Textbook Question
Calculate [OH-] and pH for each of the following strong base solutions: (c) 10.0 mL of 0.0105 M Ca(OH)2 diluted to 500.0 mL

 Verified step by step guidance
Verified step by step guidance


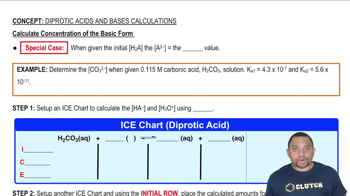
Calculate [OH-] and pH for each of the following strong base solutions: (c) 10.0 mL of 0.0105 M Ca(OH)2 diluted to 500.0 mL
Addition of phenolphthalein to an unknown colorless solution does not cause a color change. The addition of bromthymol blue to the same solution leads to a yellow color. (c) What other indicator or indicators would you want to use to determine the pH of the solution more precisely?
Calculate the pH of each of the following strong acid solutions: (b) 0.225 g of HClO3 in 2.00 L of solution