A solution is made by adding 0.300 g Ca1OH221s2, 50.0 mL of 1.40 M HNO3, and enough water to make a final volume of 75.0 mL. Assuming that all of the solid dissolves, what is the pH of the final solution?
Ch.16 - Acid-Base Equilibria

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 16, Problem 103
Calculate the pH of a solution made by adding 2.50 g of lithium oxide 1Li2O2 to enough water to make 1.500 L of solution.
 Verified step by step guidance
Verified step by step guidance1
Determine the molar mass of lithium oxide (Li2O). Calculate it by adding the atomic masses of lithium (Li) and oxygen (O) from the periodic table.
Convert the mass of lithium oxide (2.50 g) to moles using the molar mass calculated in the previous step.
Write the balanced chemical equation for the reaction of lithium oxide with water. Li2O reacts with water to form lithium hydroxide (LiOH).
Calculate the concentration of lithium hydroxide (LiOH) in the solution by dividing the moles of LiOH by the volume of the solution in liters (1.500 L).
Use the concentration of LiOH to find the pH. Since LiOH is a strong base, it dissociates completely in water, and the concentration of OH- ions is equal to the concentration of LiOH. Calculate the pOH and then use the relation pH + pOH = 14 to find the pH.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is considered neutral, while values below 7 indicate acidity and values above 7 indicate basicity. The pH is calculated as the negative logarithm of the hydrogen ion concentration in the solution, which is crucial for understanding the solution's chemical behavior.
Recommended video:
Guided course

The pH Scale
Lithium Oxide (Li2O) Dissociation
Lithium oxide is a basic oxide that reacts with water to form lithium hydroxide (LiOH). This reaction increases the concentration of hydroxide ions (OH-) in the solution, which in turn affects the pH. Understanding this dissociation is essential for calculating the resulting pH after the oxide is dissolved in water.
Recommended video:
Guided course
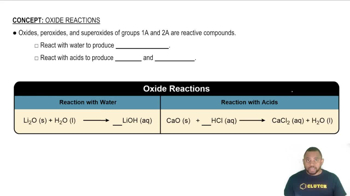
Oxide Reactions
Molarity and Concentration Calculations
Molarity is a measure of concentration defined as the number of moles of solute per liter of solution. To calculate the pH, one must first determine the number of moles of lithium oxide in the given mass and then find the concentration of hydroxide ions produced in the solution. This step is critical for accurately determining the pH of the resulting solution.
Recommended video:
Guided course

Molar Mass Calculation Example
Related Practice
Textbook Question
Textbook Question
Which, if any, of the following statements are true? (a) The stronger the base, the smaller the pKb. (b) The stronger the base, the larger the pKb. (c) The stronger the base, the smaller the Kb. (d) The stronger the base, the larger the Kb. (e) The stronger the base, the smaller the pKa of its conjugate acid. (f) The stronger the base, the larger the pKa of its conjugate acid.
Textbook Question
Predict how each molecule or ion would act, in the Brønsted-Lowry sense, in aqueous solution by writing 'acid,' 'base,' 'both,' or 'neither' on the line provided. (b) Prozac
