Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (d) decrease the volume of the container in which the reaction occurs

How do the following changes affect the value of the equilibrium constant for a gas-phase exothermic reaction: (a) removal of a reactant, (b) removal of a product?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Equilibrium Constant (K)

Le Chatelier's Principle

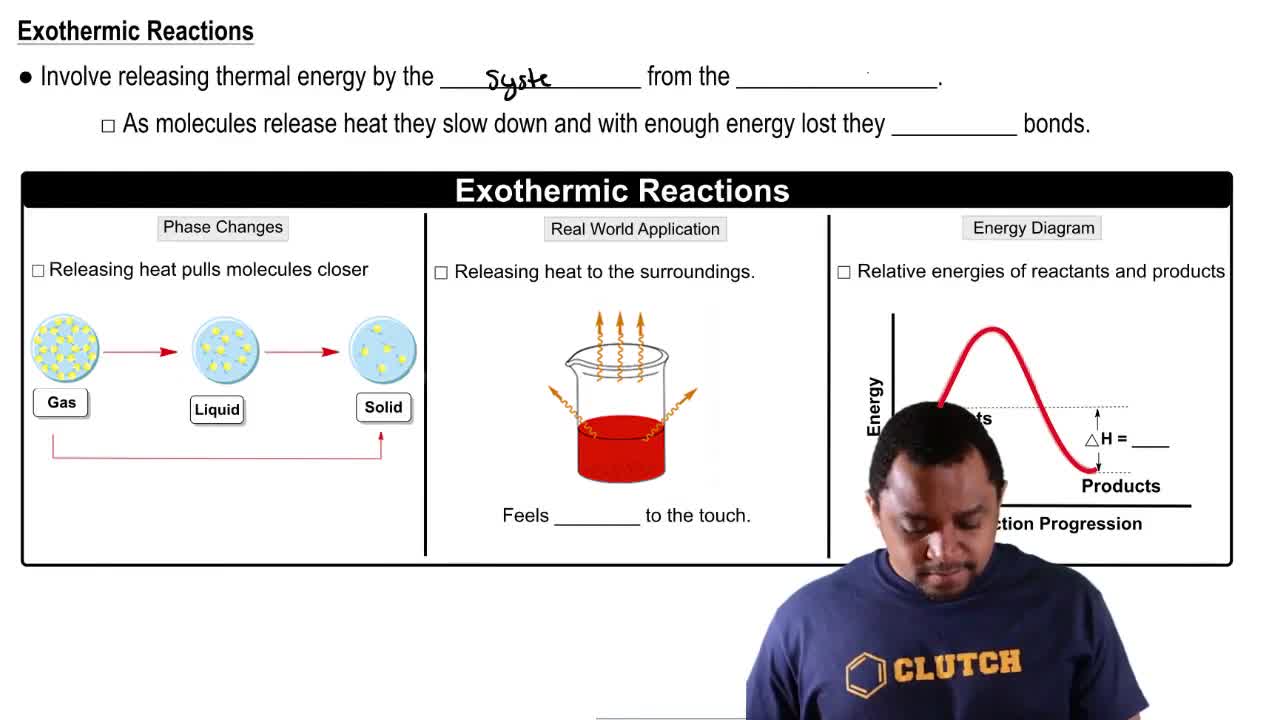
Exothermic Reactions
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (e) add a catalyst
Consider the reaction 4 NH3(𝑔) + 5 O2(𝑔) ⇌ 4 NO(𝑔) + 6 H2O(𝑔), Δ𝐻 = −904.4 kJ Does each of the following increase, decrease, or leave unchanged the yield of NO at equilibrium? (f) increase temperature.
Consider the following equilibrium between oxides of nitrogen 3 NO(g) ⇌ NO2(g) + N2O(g) (a) At constant temperature, would a change in the volume of the container affect the fraction of products in the equilibrium mixture?
Methanol (CH3OH) can be made by the reaction of CO with H2: CO(𝑔) + 2 H2(𝑔) ⇌ CH3OH(𝑔) (a) Use thermochemical data in Appendix C to calculate ΔH° for this reaction.
