Methanol (CH3OH) can be made by the reaction of CO with H2: CO(𝑔) + 2 H2(𝑔) ⇌ CH3OH(𝑔) (b) To maximize the equilibrium yield of methanol, would you use a high or low temperature?
Ch.15 - Chemical Equilibrium

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 15, Problem 66a
Methanol (CH3OH) can be made by the reaction of CO with H2: CO(𝑔) + 2 H2(𝑔) ⇌ CH3OH(𝑔) (a) Use thermochemical data in Appendix C to calculate ΔH° for this reaction.
 Verified step by step guidance
Verified step by step guidance1
Identify the standard enthalpy of formation (ΔH_f°) for each compound involved in the reaction from Appendix C.
Write the balanced chemical equation for the reaction: CO(g) + 2 H_2(g) ⇌ CH_3OH(g).
Use the formula for the standard enthalpy change of the reaction: ΔH° = Σ(ΔH_f° of products) - Σ(ΔH_f° of reactants).
Substitute the ΔH_f° values for CH_3OH(g), CO(g), and H_2(g) into the formula. Remember that the ΔH_f° for elements in their standard state, like H_2(g), is zero.
Calculate the sum of the enthalpies for the products and the reactants separately, then find the difference to determine ΔH° for the reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Thermochemical Data
Thermochemical data includes information about the enthalpy changes associated with chemical reactions. This data is often found in tables and can provide standard enthalpy of formation values (ΔH°f) for various substances. By using these values, one can calculate the overall enthalpy change (ΔH°) for a reaction by applying Hess's law, which states that the total enthalpy change is the sum of the enthalpy changes for individual steps.
Recommended video:
Guided course
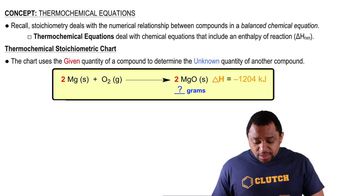
Thermochemical Equations
Hess's Law
Hess's Law states that the total enthalpy change for a chemical reaction is the same, regardless of the number of steps or the pathway taken. This principle allows chemists to calculate the enthalpy change of a reaction by summing the enthalpy changes of individual reactions that lead to the same products. It is particularly useful when direct measurement of ΔH° is difficult or impossible.
Recommended video:
Guided course
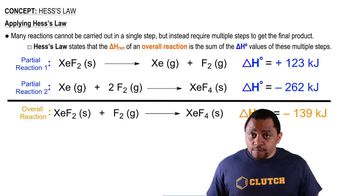
Hess's Law
Standard Enthalpy of Formation (ΔH°f)
The standard enthalpy of formation (ΔH°f) is defined as the change in enthalpy when one mole of a compound is formed from its elements in their standard states. This value is crucial for calculating the enthalpy change of a reaction using the formula ΔH° = ΣΔH°f(products) - ΣΔH°f(reactants). Understanding how to use ΔH°f values allows for the determination of the energy changes involved in chemical reactions.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
Textbook Question
Consider the following equilibrium between oxides of nitrogen 3 NO(g) ⇌ NO2(g) + N2O(g) (a) At constant temperature, would a change in the volume of the container affect the fraction of products in the equilibrium mixture?
Textbook Question
Methanol (CH3OH) can be made by the reaction of CO with H2: CO(𝑔) + 2 H2(𝑔) ⇌ CH3OH(𝑔) (c) To maximize the equilibrium yield of methanol, would you use a high or low pressure?
Textbook Question
Ozone, O3, decomposes to molecular oxygen in the stratosphere according to the reaction 2 O3(𝑔) ⟶ 3 O2(𝑔). Would increasing the pressure by decreasing the size of the reaction vessel favor the formation of ozone or of oxygen?
