A sample of nitrosyl bromide (NOBr) decomposes according to the equation 2 NOBr(𝑔) ⇌ 2 NO(𝑔) + Br2(𝑔) An equilibrium mixture in a 5.00-L vessel at 100°C contains 3.22 g of NOBr, 2.46 g of NO, and 6.55 g of Br2. (c) What was the mass of the original sample of NOBr?

For the equilibrium 2 IBr(g) ⇌ I2(g) + Br2(g), Kp = 8.5 * 10^-3 at 150 _x001F_C. If 0.025 atm of IBr is placed in a 2.0-L container, what is the partial pressure of all substances after equilibrium is reached?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
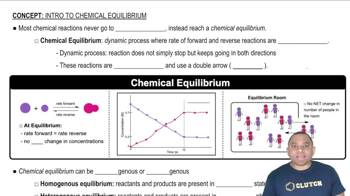
Chemical Equilibrium
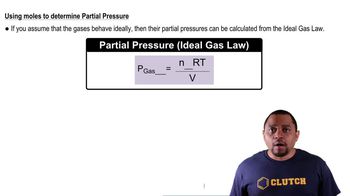
Partial Pressure
Equilibrium Constant (Kp)

Consider the hypothetical reaction A(𝑔) ⇌ 2 B(𝑔). A flask is charged with 0.75 atm of pure A, after which it is allowed to reach equilibrium at 0°C. At equilibrium, the partial pressure of A is 0.36 atm. (c) To maximize the yield of product B, would you make the reaction flask larger or smaller?
As shown in Table 15.2, the equilibrium constant for the reaction N2(g) + 3 H2(g) ⇌ 2 NH3(g) is Kp = 4.34 × 10-3 at 300°C. Pure NH3 is placed in a 1.00-L flask and allowed to reach equilibrium at this temperature. There are 1.05 g NH3 in the equilibrium mixture. (b) What was the initial mass of ammonia placed in the vessel?
For the equilibrium PH3BCl3(𝑠) ⇌ PH3(𝑔) + BCl3(𝑔) 𝐾𝑝 = 0.052 at 60°C. (b) A closed 1.500-L vessel at 60°C is charged with 0.0500 g of BCl3(𝑔); 3.00 g of solid PH3BCl3 is then added to the flask, and the system is allowed to equilibrate. What is the equilibrium concentration of PH3?
A 0.831-g sample of SO3 is placed in a 1.00-L container and heated to 1100 K. The SO3 decomposes to SO2 and O2: 2SO3(𝑔) ⇌ 2 SO2(𝑔) + O2(𝑔) At equilibrium, the total pressure in the container is 1.300 atm. Find the values of 𝐾𝑝 and 𝐾𝑐 for this reaction at 1100 K.
