When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (a) Calculate Kc for this reaction at this temperature.

A sample of nitrosyl bromide (NOBr) decomposes according to the equation 2 NOBr(𝑔) ⇌ 2 NO(𝑔) + Br2(𝑔) An equilibrium mixture in a 5.00-L vessel at 100°C contains 3.22 g of NOBr, 2.46 g of NO, and 6.55 g of Br2. (b) What is the total pressure exerted by the mixture of gases?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ideal Gas Law

Molar Mass and Mass to Moles Conversion

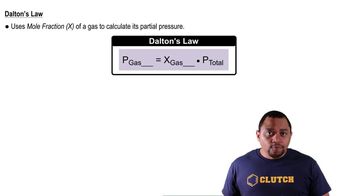
Dalton's Law of Partial Pressures
When 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2: SO2Cl2(g) ⇌ SO2(g) + Cl2(g) (c) According to Le Châtelier's principle, would the percent of SO2Cl2 that decomposes increase, decrease or stay the same if the mixture were transferred to a 15.00-L vessel?
A sample of nitrosyl bromide (NOBr) decomposes according to the equation 2 NOBr(𝑔) ⇌ 2 NO(𝑔) + Br2(𝑔) An equilibrium mixture in a 5.00-L vessel at 100°C contains 3.22 g of NOBr, 2.46 g of NO, and 6.55 g of Br2. (c) What was the mass of the original sample of NOBr?
Consider the hypothetical reaction A(𝑔) ⇌ 2 B(𝑔). A flask is charged with 0.75 atm of pure A, after which it is allowed to reach equilibrium at 0°C. At equilibrium, the partial pressure of A is 0.36 atm. (c) To maximize the yield of product B, would you make the reaction flask larger or smaller?
As shown in Table 15.2, the equilibrium constant for the reaction N2(g) + 3 H2(g) ⇌ 2 NH3(g) is Kp = 4.34 × 10-3 at 300°C. Pure NH3 is placed in a 1.00-L flask and allowed to reach equilibrium at this temperature. There are 1.05 g NH3 in the equilibrium mixture. (b) What was the initial mass of ammonia placed in the vessel?
