Textbook Question
A mixture of 0.2000 mol of CO2, 0.1000 mol of H2, and 0.1600 mol of H2O is placed in a 2.000-L vessel. The following equilibrium is established at 500 K: CO2(g) + H2(g) ⇌ CO(g) + H2O(g) (d) Calculate Kc for the reaction.
1
views

 Verified step by step guidance
Verified step by step guidance
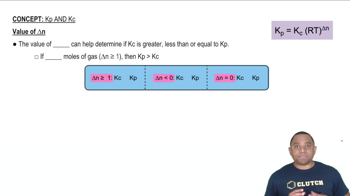

A mixture of 0.2000 mol of CO2, 0.1000 mol of H2, and 0.1600 mol of H2O is placed in a 2.000-L vessel. The following equilibrium is established at 500 K: CO2(g) + H2(g) ⇌ CO(g) + H2O(g) (d) Calculate Kc for the reaction.
Indicate whether each of the following statements about the reaction quotient Q is true or false: (a) The expression for 𝑄𝑐 looks the same as the expression for 𝐾𝑐.
Indicate whether each of the following statements about the reaction quotient Q is true or false: (b) If 𝑄𝑐 < 𝐾𝑐, the reaction needs to proceed to the right to reach equilibrium.