As described in Exercise 14.41, the decomposition of sulfuryl chloride (SO2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 × 10-2 s-1. (b) At what time will the partial pressure of SO2Cl2 decline to one-tenth its initial value?

The reaction SO2Cl2(g) → SO2(g) + Cl2(g) is first order in SO2Cl2. Using the following kinetic data, determine the magnitude and units of the first-order rate constant: Time (s) Pressure SO2Cl2 (atm) 0 1.000 2500 0.947 5000 0.895 7500 0.848 10,000 0.803
 Verified step by step guidance
Verified step by step guidanceKey Concepts
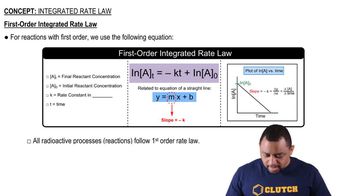
First-Order Reactions
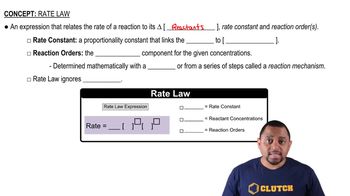
Rate Constant (k)

Integrated Rate Law
The first-order rate constant for the decomposition of N2O5, 2 N2O5(g) → 4 NO2(g) + O2(g), at 70°C is 6.82×10-3 s-1. Suppose we start with 0.0250 mol of N2O5(g) in a volume of 2.0 L. (a) How many moles of N2O5 will remain after 5.0 min?
The first-order rate constant for the decomposition of N2O5, 2 N2O5(g) → 4 NO2(g) + O2(g), at 70°C is 6.82×10-3 s-1. Suppose we start with 0.0250 mol of N2O5(g) in a volume of 2.0 L. (c) What is the half-life of N2O5 at 70°C?
From the following data for the first-order gas-phase isomerization of CH3NC to CH3CN at 215°C, calculate the first-order rate constant and half-life for the reaction:
Time (s) Pressure CH3NC (torr)
0 502
2000 335
5000 180
8000 95.5
12,000 41.7
15,000 22.4
Consider the data presented in Exercise 14.19. (a) By using appropriate graphs, determine whether the reaction is first order or second order.
Consider the data presented in Exercise 14.19. (c) What is the half-life for the reaction?
