(a) The activation energy for the isomerization of methyl isonitrile (Figure 14.6) is 160 kJ>mol. Calculate the fraction of methyl isonitrile molecules that has an energy equal to or greater than the activation energy at 500 K. (b) Calculate this fraction for a temperature of 520 K. What is the ratio of the fraction at 520 K to that at 500 K?
Ch.14 - Chemical Kinetics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 14, Problem 55a
The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction, and label Ea and ΔE.
 Verified step by step guidance
Verified step by step guidance1
Identify the key components to be included in the energy profile diagram: reactants, products, activation energy (Ea), and overall energy change (ΔE).
Start by drawing a horizontal line representing the energy level of the reactants. Label this line as 'Reactants'.
Above the reactants line, mark a point that represents the peak energy level the reaction must reach before proceeding to products. This point is the activation energy (Ea). Label this point and draw an arrow from the reactants line to this point, indicating the energy input needed to reach this state.
From the peak, draw a line descending to the energy level of the products, which is lower than that of the reactants by 66 kJ, as given by the overall energy change (ΔE). Label this line as 'Products'.
On the diagram, clearly label the activation energy (Ea) as 7 kJ above the reactants' energy and the overall energy change (ΔE) as -66 kJ below the reactants' energy.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Energy Profile Diagrams
Energy profile diagrams visually represent the energy changes during a chemical reaction. They plot the energy of the system against the progress of the reaction, showing the energy of reactants, products, and the activation energy (Ea). The diagram helps illustrate whether a reaction is exothermic or endothermic, as well as the energy barrier that must be overcome for the reaction to proceed.
Recommended video:
Guided course
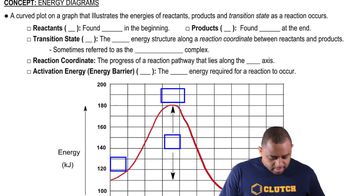
Energy Diagrams
Activation Energy (Ea)
Activation energy is the minimum energy required for reactants to undergo a chemical reaction. It represents the energy barrier that must be overcome for the reaction to proceed. In the given reaction, the activation energy is 7 kJ, indicating that this amount of energy must be supplied to initiate the reaction, regardless of whether the overall reaction is exothermic or endothermic.
Recommended video:
Guided course
Activity Series Chart
Enthalpy Change (ΔE)
The enthalpy change (ΔE) of a reaction indicates the difference in energy between the reactants and products. A negative ΔE, such as -66 kJ in this case, signifies that the reaction releases energy to the surroundings, classifying it as exothermic. This value is crucial for understanding the thermodynamics of the reaction and its favorability under standard conditions.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
1
views
Textbook Question
Calculate the fraction of atoms in a sample of argon gas at 400 K that has an energy of 10.0 kJ or greater.
Textbook Question
The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (b) What is the activation energy for the reverse reaction?
Textbook Question
Indicate whether each statement is true or false. (c) Increasing the reaction temperature increases the fraction of successful collisions between reactants.
1
views
