(a) The activation energy for the isomerization of methyl isonitrile (Figure 14.6) is 160 kJ>mol. Calculate the fraction of methyl isonitrile molecules that has an energy equal to or greater than the activation energy at 500 K. (b) Calculate this fraction for a temperature of 520 K. What is the ratio of the fraction at 520 K to that at 500 K?
Ch.14 - Chemical Kinetics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 14, Problem 53
Calculate the fraction of atoms in a sample of argon gas at 400 K that has an energy of 10.0 kJ or greater.
 Verified step by step guidance
Verified step by step guidance1
Identify the relevant concept: The problem involves calculating the fraction of atoms with a certain energy in a gas, which is related to the Maxwell-Boltzmann distribution.
Use the Maxwell-Boltzmann distribution formula: \( f(E) = \frac{2}{\sqrt{\pi}} \left( \frac{E}{kT} \right)^{1/2} e^{-E/kT} \), where \( E \) is the energy, \( k \) is the Boltzmann constant, and \( T \) is the temperature in Kelvin.
Convert the given energy from kJ to J: Since 1 kJ = 1000 J, convert 10.0 kJ to Joules.
Calculate the exponent in the Maxwell-Boltzmann distribution: \( -E/kT \), where \( k = 1.38 \times 10^{-23} \text{ J/K} \) and \( T = 400 \text{ K} \).
Integrate the Maxwell-Boltzmann distribution from 10.0 kJ to infinity to find the fraction of atoms with energy greater than or equal to 10.0 kJ.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Maxwell-Boltzmann Distribution
The Maxwell-Boltzmann distribution describes the distribution of speeds (or energies) of particles in a gas at a given temperature. It shows that at higher temperatures, a greater fraction of particles have higher energies. This concept is essential for understanding how many argon atoms will have energies above a certain threshold, such as 10.0 kJ, at 400 K.
Recommended video:
Guided course
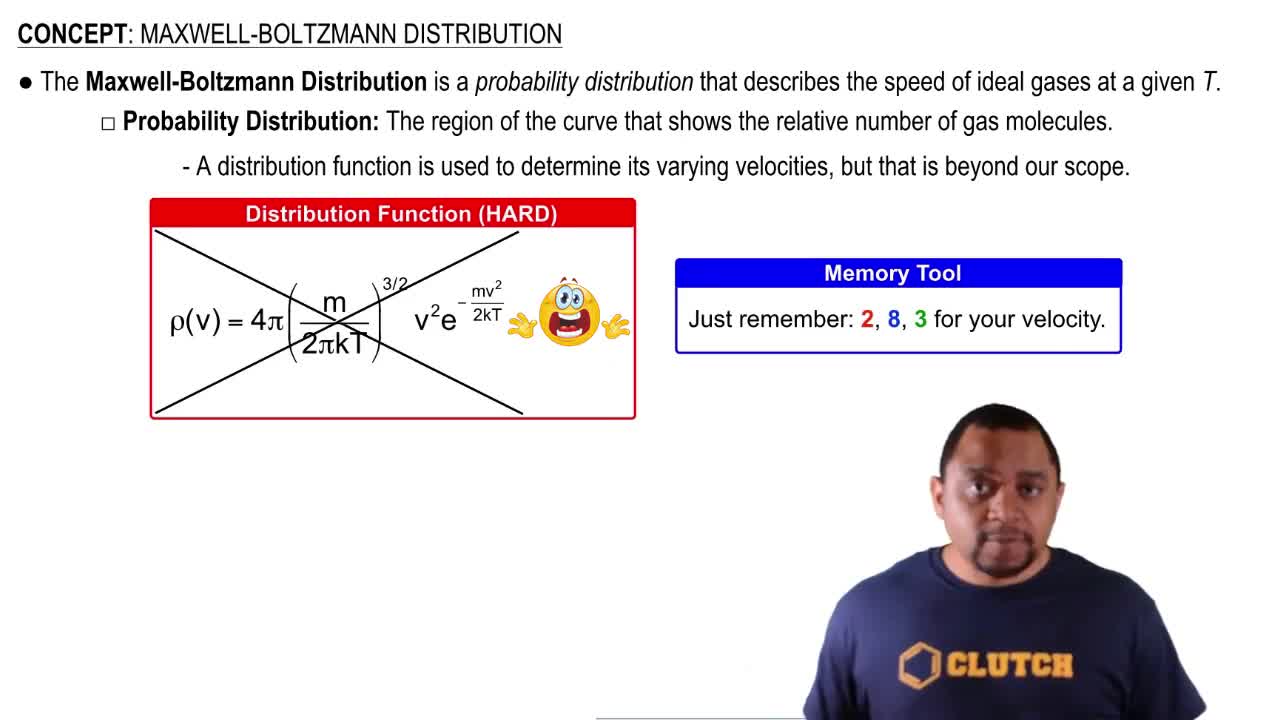
Maxwell-Boltzmann Distribution
Kinetic Energy and Temperature Relationship
The kinetic energy of gas particles is directly related to the temperature of the gas, as described by the equation KE = (3/2)kT for monatomic gases, where k is the Boltzmann constant and T is the temperature in Kelvin. This relationship helps in calculating the average energy of argon atoms at 400 K and determining the fraction that meets or exceeds the specified energy level.
Recommended video:
Guided course
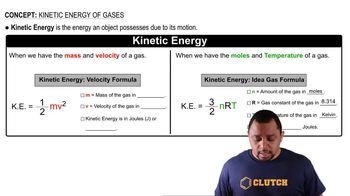
Kinetic Energy Formulas
Boltzmann Factor
The Boltzmann factor, given by e^(-E/kT), quantifies the probability of a particle having energy E at temperature T. It is crucial for calculating the fraction of argon atoms with energies equal to or greater than 10.0 kJ. By applying this factor, one can derive the proportion of atoms that possess sufficient energy to exceed the given threshold.
Recommended video:
Guided course

Maxwell-Boltzmann Distribution
Related Practice
Textbook Question
1
views
Textbook Question
(a) What factors determine whether a collision between two molecules will lead to a chemical reaction?
1
views
Textbook Question
(b) Does the rate constant for a reaction generally increase or decrease with an increase in reaction temperature?
1
views
Textbook Question
The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (a) Sketch the energy profile for the reaction, and label Ea and ΔE.
Textbook Question
The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (b) What is the activation energy for the reverse reaction?
