The gas-phase reaction Cl(g) + HBr(g) → HCl(g) + Br(g) has an overall energy change of -66 kJ. The activation energy for the reaction is 7 kJ. (b) What is the activation energy for the reverse reaction?
Ch.14 - Chemical Kinetics

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 14, Problem 58a
Indicate whether each statement is true or false. (a) If you measure the rate constant for a reaction at different temperatures, you can calculate the overall enthalpy change for the reaction.
 Verified step by step guidance
Verified step by step guidance1
Identify the relationship between the rate constant (k) and temperature, which is described by the Arrhenius equation: \( k = A e^{-\frac{E_a}{RT}} \), where \( A \) is the pre-exponential factor, \( E_a \) is the activation energy, \( R \) is the gas constant, and \( T \) is the temperature in Kelvin.
Understand that the enthalpy change (\( \Delta H \)) of a reaction is different from the activation energy (\( E_a \)). The activation energy is the energy barrier that must be overcome for reactants to transform into products, while the enthalpy change is the overall heat absorbed or released during the reaction.
Recognize that measuring the rate constant at different temperatures primarily provides information about the activation energy through the Arrhenius plot, where plotting \( ln(k) \) versus \( \frac{1}{T} \) gives a slope of \( -\frac{E_a}{R} \).
Conclude that while the activation energy can be derived from the temperature dependence of the rate constant, the overall enthalpy change of the reaction cannot be directly calculated from this data alone, as it requires additional thermodynamic information such as the heat capacities of reactants and products.
Determine the statement as false, since the rate constant measurements at different temperatures allow calculation of the activation energy, not the direct calculation of the overall enthalpy change of the reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Rate Constant and Temperature
The rate constant (k) of a chemical reaction is temperature-dependent, typically increasing with temperature due to higher kinetic energy of molecules. This relationship is described by the Arrhenius equation, which shows how k varies with temperature and activation energy. Understanding this concept is crucial for analyzing how temperature influences reaction rates.
Recommended video:
Guided course
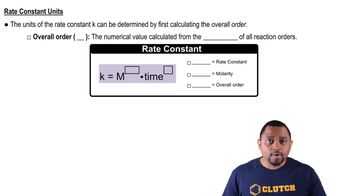
Rate Constant Units
Enthalpy Change (ΔH)
Enthalpy change (ΔH) is a measure of the heat absorbed or released during a chemical reaction at constant pressure. It reflects the difference in energy between reactants and products. While the rate constant can provide insights into reaction kinetics, ΔH is determined through thermodynamic principles, often requiring calorimetric data or Hess's law for accurate calculation.
Recommended video:
Guided course

Enthalpy of Formation
Van 't Hoff Equation
The Van 't Hoff equation relates the change in the equilibrium constant of a reaction to the change in temperature and the enthalpy change of the reaction. It allows for the calculation of ΔH if the equilibrium constants at two different temperatures are known. This equation is essential for linking thermodynamics and kinetics, particularly in the context of temperature effects on reaction rates.
Recommended video:
Guided course

Van der Waals Equation
Related Practice
Textbook Question
Textbook Question
Indicate whether each statement is true or false. (c) Increasing the reaction temperature increases the fraction of successful collisions between reactants.
1
views
Textbook Question
Indicate whether each statement is true or false. (b) Exothermic reactions are faster than endothermic reactions.
Textbook Question
Indicate whether each statement is true or false. (c) If you double the temperature for a reaction, you cut the activation energy in half.
1
views
Textbook Question
Based on their activation energies and energy changes and assuming that all collision factors are the same, rank the following reactions from slowest to fastest. (a) Ea = 45 kJ>mol; E = -25 kJ>mol (b) Ea = 35 kJ>mol; E = -10 kJ>mol (c) Ea = 55 kJ>mol; E = 10 kJ>mol
