The reaction between ethyl iodide and hydroxide ion in ethanol (C2H5OH) solution, C2H5I(alc) + OH-(alc) → C2H5OH(l) + I-(alc), has an activation energy of 86.8 kJ/mol and a frequency factor of 2.10 × 1011 M-1 s-1. (c) Which reagent in the reaction is limiting, assuming the reaction proceeds to completion?

Enzymes are often described as following the two-step mechanism:
E + S ⇌ ES (fast)
ES → E + P (slow)
where E = enzyme, S = substrate, ES = enzyme9substrate complex, and P = product.
(b) Molecules that can bind to the active site of an enzyme but are not converted into product are called enzyme inhibitors. Write an additional elementary step to add into the preceding mechanism to account for the reaction of E with I, an inhibitor.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Enzyme-Substrate Complex Formation
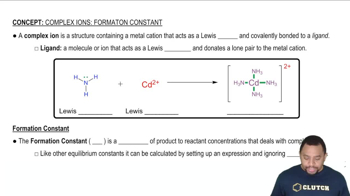
Enzyme Inhibition
Reaction Mechanism
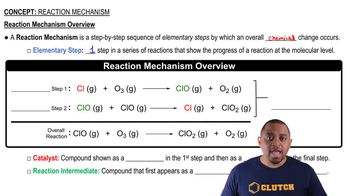
One of the many remarkable enzymes in the human body is carbonic anhydrase, which catalyzes the interconversion of carbon dioxide and water with bicarbonate ion and protons. If it were not for this enzyme, the body could not rid itself rapidly enough of the CO2 accumulated by cell metabolism. The enzyme catalyzes the dehydration (release to air) of up to 107 CO2 molecules per second. Which components of this description correspond to the terms enzyme, substrate, and turnover number?
Enzymes are often described as following the two-step mechanism:
E + S ⇌ ES (fast)
ES → E + P (slow)
where E = enzyme, S = substrate, ES = enzyme9substrate complex, and P = product.
(a) If an enzyme follows this mechanism, what rate law is expected for the reaction?
The reaction between ethyl iodide and hydroxide ion in ethanol (C2H5OH) solution, C2H5I(alc) + OH-(alc) → C2H5OH(l) + I-(alc), has an activation energy of 86.8 kJ/mol and a frequency factor of 2.10 × 1011 M-1 s-1. (d) Assuming the frequency factor and activation energy do not change as a function of temperature, calculate the rate constant for the reaction at 50 C.
