Consider two ionic solids, both composed of singly charged ions, that have different lattice energies. (a) Will the solids have the same solubility in water? (b) If not, which solid will be more soluble in water, the one with the larger lattice energy or the one with the smaller lattice energy? Assume that solute–solvent interactions are the same for both solids. [Section 13.1]
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 7
The structures of vitamins E and B6 are shown below. Predict which is more water soluble and which is more fat soluble. [Section 13.3]
 Verified step by step guidance
Verified step by step guidance1
Identify the key structural features of vitamins E and B6 that influence their solubility, focusing on the presence of polar (hydrophilic) groups and nonpolar (hydrophobic) regions.
Recall that water solubility is generally associated with molecules containing polar functional groups such as hydroxyl (-OH), amino (-NH2), or phosphate groups, which can form hydrogen bonds with water.
Recognize that fat solubility is favored by large nonpolar hydrocarbon chains or rings, which interact well with lipid environments but poorly with water.
Examine vitamin E's structure for long hydrocarbon chains or aromatic rings that contribute to its nonpolar character, making it more fat soluble.
Examine vitamin B6's structure for multiple polar groups (such as hydroxyl and amino groups) that increase its ability to interact with water, making it more water soluble.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility and Polarity
Solubility depends on the polarity of molecules; polar molecules dissolve well in water (a polar solvent), while nonpolar molecules dissolve better in fats or oils (nonpolar solvents). Understanding the presence of polar groups like hydroxyl or amine groups helps predict solubility.
Recommended video:
Guided course

Molecular Polarity
Structure and Functional Groups of Vitamins
The chemical structure and functional groups of vitamins determine their polarity. Vitamin B6 contains polar groups such as hydroxyl and amine groups, making it more water soluble, whereas vitamin E has a long nonpolar hydrocarbon chain, increasing its fat solubility.
Recommended video:
Guided course
Functional Groups Example
Hydrophobic vs. Hydrophilic Interactions
Hydrophobic molecules repel water and tend to dissolve in lipids, while hydrophilic molecules attract water and dissolve in aqueous environments. Recognizing these interactions helps explain why some vitamins are fat soluble (like vitamin E) and others are water soluble (like vitamin B6).
Recommended video:
Guided course
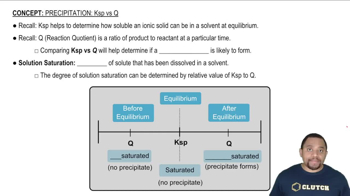
Ksp vs Q in Precipitation
Related Practice
Textbook Question
3
views
Textbook Question
The figure shows two identical volumetric flasks containing the same solution at two temperatures. (b) Does the molality of the solution change with the change in temperature? [Section 13.4]
1
views
Textbook Question
This portion of a phase diagram shows the vapor–pressure curves of a volatile solvent and of a solution of that solvent containing a nonvolatile solute. (b) What are the normal boiling points of the solvent and the solution? [Section 13.5]
1
views
