This figure shows the interaction of a cation with surrounding water molecules. (b) Which of the following explanations accounts for the fact that the ion–solvent interaction is greater for Li+ than for K+? a. Li+ is of lower mass than K+. b. The ionization energy of Li is higher than that for K. c. Li+ has a smaller ionic radius than K+. d. Li has a lower density than K. e. Li reacts with water more slowly than K. [Section 13.1]
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 6
If you compare the solubilities of the noble gases in water, you find that solubility increases from smallest atomic weight to largest, specifically: Ar < Kr < Xe. Which of the following statements is the best explanation? [Section 13.3] (a) The heavier the gas, the more it sinks to the bottom of the water and leaves room for more gas molecules at the top of the water. (b) The heavier the gas, the more dispersion forces it has, and therefore the more attractive interactions it has with water molecules. (c) The heavier the gas, the more likely it is to hydrogen-bond with water. (d) The heavier the gas, the more likely it is to make a saturated solution in water.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the nature of noble gases. Noble gases are nonpolar and do not form hydrogen bonds with water. This eliminates option (c) as a possible explanation.
Step 2: Consider the role of dispersion forces. Dispersion forces, also known as London dispersion forces, increase with the size and mass of the atoms or molecules. Larger noble gases have more electrons, which can lead to stronger dispersion forces.
Step 3: Evaluate the effect of dispersion forces on solubility. Stronger dispersion forces can lead to greater interactions between the noble gas atoms and water molecules, potentially increasing solubility.
Step 4: Analyze the incorrectness of other options. Option (a) is incorrect because solubility is not about gases sinking or floating, but about their ability to dissolve. Option (d) is incorrect because saturation is not directly related to atomic weight.
Step 5: Conclude that option (b) is the best explanation. The increase in dispersion forces with heavier noble gases leads to stronger attractive interactions with water, thus increasing solubility.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility and Atomic Weight
Solubility refers to the ability of a substance to dissolve in a solvent, such as water. In the case of noble gases, their solubility tends to increase with atomic weight due to the greater number of electrons, which enhances the strength of dispersion forces. This trend indicates that heavier noble gases can interact more effectively with water molecules, leading to higher solubility.
Recommended video:
Guided course

Solubility Rules
Dispersion Forces
Dispersion forces, also known as London dispersion forces, are weak intermolecular forces that arise from temporary fluctuations in electron density within molecules. For noble gases, as atomic weight increases, the number of electrons also increases, resulting in stronger dispersion forces. This increased interaction with water molecules contributes to the higher solubility of heavier noble gases.
Recommended video:
Guided course
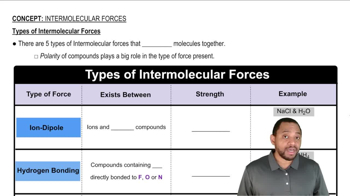
Types of Intermolecular Forces
Hydrogen Bonding
Hydrogen bonding is a strong type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms like oxygen or nitrogen. While noble gases do not form hydrogen bonds with water, understanding this concept is crucial for distinguishing between types of interactions. The solubility of noble gases is primarily influenced by dispersion forces rather than hydrogen bonding, which is not applicable in this context.
Recommended video:
Guided course

Hydrogenation Reactions
Related Practice
Textbook Question
Textbook Question
Consider two ionic solids, both composed of singly charged ions, that have different lattice energies. (a) Will the solids have the same solubility in water? (b) If not, which solid will be more soluble in water, the one with the larger lattice energy or the one with the smaller lattice energy? Assume that solute–solvent interactions are the same for both solids. [Section 13.1]
3
views
Textbook Question
The structures of vitamins E and B6 are shown below. Predict which is more water soluble and which is more fat soluble. [Section 13.3]
Textbook Question
The figure shows two identical volumetric flasks containing the same solution at two temperatures. (b) Does the molality of the solution change with the change in temperature? [Section 13.4]
1
views
