Textbook Question
Indicate the type of solute–solvent interaction that should be most important in each of the following solutions: a. CCl4 in benzene (C6H6),

 Verified step by step guidance
Verified step by step guidance
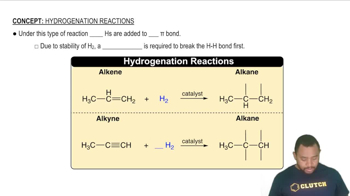
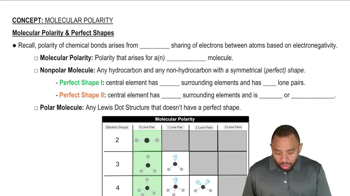
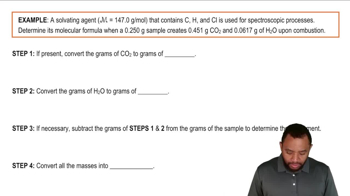
Indicate the type of solute–solvent interaction that should be most important in each of the following solutions: a. CCl4 in benzene (C6H6),
Indicate the type of solute–solvent interaction that should be most important in each of the following solutions: c. KBr in water,
Indicate the type of solute–solvent interaction that should be most important in each of the following solutions: d. HCl in acetonitrile (CH3CN).
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (a) KCl in water