Most fish need at least 4 ppm dissolved O2 in water for survival. (b) What partial pressure of O2 above water is needed to obtain 4 ppm O2 in water at 10 °C? (The Henry's law constant for O2 at this temperature is 1.71⨉10-3 mol/L-atm.)

Glucose makes up about 0.10% by mass of human blood. Calculate this concentration in molality. What further information would you need to determine the molarity of the solution?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
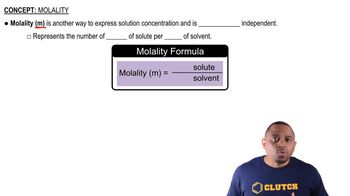
Molality
Molarity

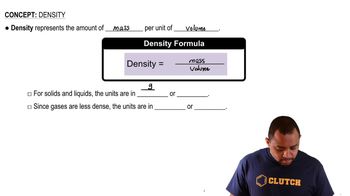
Density of the Solution
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (a) Assuming that the solubility of radon in water with 1 atm pressure of the gas over the water at 30 °C is 7.27⨉10-3 M, what is the Henry's law constant for radon in water at this temperature?
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (b) A sample consisting of various gases contains 3.5 × 10-6 mole fraction of radon. This gas at a total pressure of 32 atm is shaken with water at 30 °C. Calculate the molar concentration of radon in the water.
The maximum allowable concentration of lead in drinking water is 9.0 ppb. (a) Calculate the molarity of lead in a 9.0-ppb solution.
The maximum allowable concentration of lead in drinking water is 9.0 ppb. (b) How many grams of lead are in a swimming pool containing 9.0 ppb lead in 60 m3 of water?
The first stage of treatment at a reverse osmosis plant is to flow the water through rock, sand, and gravel as shown here. Would this step remove particulate matter? Would this step remove dissolved salts?
