By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution: (d) Pb(NO3)2.

Consider water and glycerol, CH2(OH)CH(OH)CH2OH. (b) List the intermolecular attractions that occur between a water molecule and a glycerol molecule.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Hydrogen Bonding

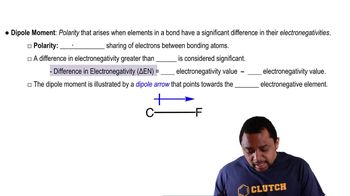
Dipole-Dipole Interactions
Van der Waals Forces

By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (c) Ce2(SO4)3.
Oil and water are immiscible. Which is the most likely reason? (a) Oil molecules are denser than water. (b) Oil molecules are composed mostly of carbon and hydrogen. (c) Oil molecules have higher molar masses than water. (d) Oil molecules have higher vapor pressures than water. (e) Oil molecules have higher boiling points than water.
Which of the following in each pair is likely to be more soluble in hexane, C6H14: (a) CCl4 or CaCl2, (b) benzene (C6H6) or glycerol, CH2(OH)CH(OH)CH2OH, (c) octanoic acid, CH3CH2CH2CH2CH2CH2CH2COOH, or acetic acid, CH3COOH? Explain your answer in each case.
