By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution:(c) K2Cr2O7
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 26b
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,
 Verified step by step guidance
Verified step by step guidance1
Identify the solubility of Pb(NO_3)_2 at 30 °C from Figure 13.15. This value is typically given in grams of solute per 100 grams of water.
Since the solubility is given per 100 grams of water, calculate the amount of Pb(NO_3)_2 needed for 250 grams of water. Use the ratio: (solubility in g/100 g water) = (x g/250 g water).
Solve the proportion to find the mass of Pb(NO_3)_2 required for 250 grams of water.
Ensure the units are consistent throughout the calculation to avoid any errors.
Double-check the solubility value from the figure to ensure accuracy in your calculations.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Saturation and Solubility
Saturation refers to the point at which a solution can no longer dissolve additional solute at a given temperature and pressure. Solubility is the maximum amount of solute that can dissolve in a specific amount of solvent at a particular temperature. Understanding these concepts is crucial for determining how much of a salt, like Pb(NO3)2, can be dissolved in water.
Recommended video:
Guided course

Solubility Rules
Molar Mass Calculation
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). To find the mass of Pb(NO3)2 needed for a saturated solution, one must first calculate its molar mass by summing the atomic masses of its constituent elements. This calculation is essential for converting between moles and grams in stoichiometric problems.
Recommended video:
Guided course

Molar Mass Calculation Example
Temperature Dependence of Solubility
The solubility of most salts varies with temperature; generally, solubility increases with rising temperature. For the question at hand, knowing that the solubility of Pb(NO3)2 at 30 °C is necessary to determine how much of the salt can be dissolved in 250 g of water. This relationship is key to solving the problem accurately.
Recommended video:
Guided course
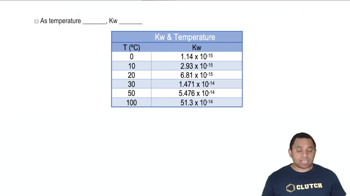
Kw Temperature Dependence
Related Practice
Textbook Question
Textbook Question
By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution: (d) Pb(NO3)2.
Textbook Question
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (c) Ce2(SO4)3.
Textbook Question
Oil and water are immiscible. Which is the most likely reason? (a) Oil molecules are denser than water. (b) Oil molecules are composed mostly of carbon and hydrogen. (c) Oil molecules have higher molar masses than water. (d) Oil molecules have higher vapor pressures than water. (e) Oil molecules have higher boiling points than water.
