Which type (or types) of crystalline solid is characterized by each of the following? (d) network of covalent bonds.

You are given a white substance that melts at 100 °C. The substance is soluble in water. Neither the solid nor the solution is a conductor of electricity. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Melting Point
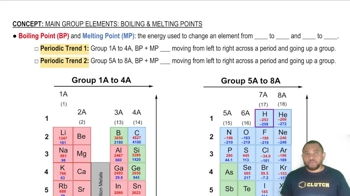
Solubility in Water

Electrical Conductivity
Indicate the type of solid (molecular, metallic, ionic, or covalent-network) for each compound: (c) Ta2O5 (melting point, 1872°C)
You are given a gray substance that melts at 700 °C; the solid is a conductor of electricity and is insoluble in water. Which type of solid (molecular, metallic, covalent-network, or ionic) might this substance be?
(a) Draw a picture that represents a crystalline solid at the atomic level.
(b) Now draw a picture that represents an amorphous solid at the atomic level.
Two patterns of packing for two different circles of the same size are shown here. For each structure (b) determine the angle between the lattice vectors, g, and determine whether the lattice vectors are of the same length or of different lengths; (i)
(ii)
