Imagine the primitive cubic lattice. Now imagine grabbing the top of it and stretching it straight up. All angles remain 90. What kind of primitive lattice have you made?
Ch.12 - Solids and Modern Materials

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 12, Problem 27
What is the minimum number of atoms that could be contained in the unit cell of an element with a body-centered cubic lattice? (a) 1, (b) 2, (c) 3, (d) 4, (e) 5.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the structure of a body-centered cubic (BCC) lattice. In a BCC lattice, atoms are located at each corner of the cube and one atom is at the center of the cube.
Step 2: Calculate the contribution of corner atoms to the unit cell. Each corner atom is shared by eight adjacent unit cells, so each corner atom contributes 1/8 of an atom to the unit cell.
Step 3: Determine the total contribution of the corner atoms. Since there are 8 corners in a cube, the total contribution from the corner atoms is 8 * (1/8) = 1 atom.
Step 4: Consider the atom at the center of the cube. This atom is not shared with any other unit cell, so it contributes fully to the unit cell.
Step 5: Add the contributions from the corner atoms and the center atom. The total number of atoms in a BCC unit cell is 1 (from corners) + 1 (from center) = 2 atoms.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Unit Cell
A unit cell is the smallest repeating unit in a crystal lattice that reflects the symmetry and structure of the entire crystal. It defines the arrangement of atoms in the crystal and can vary in shape and size. Understanding the unit cell is crucial for determining the properties of the material, including its density and packing efficiency.
Recommended video:
Guided course
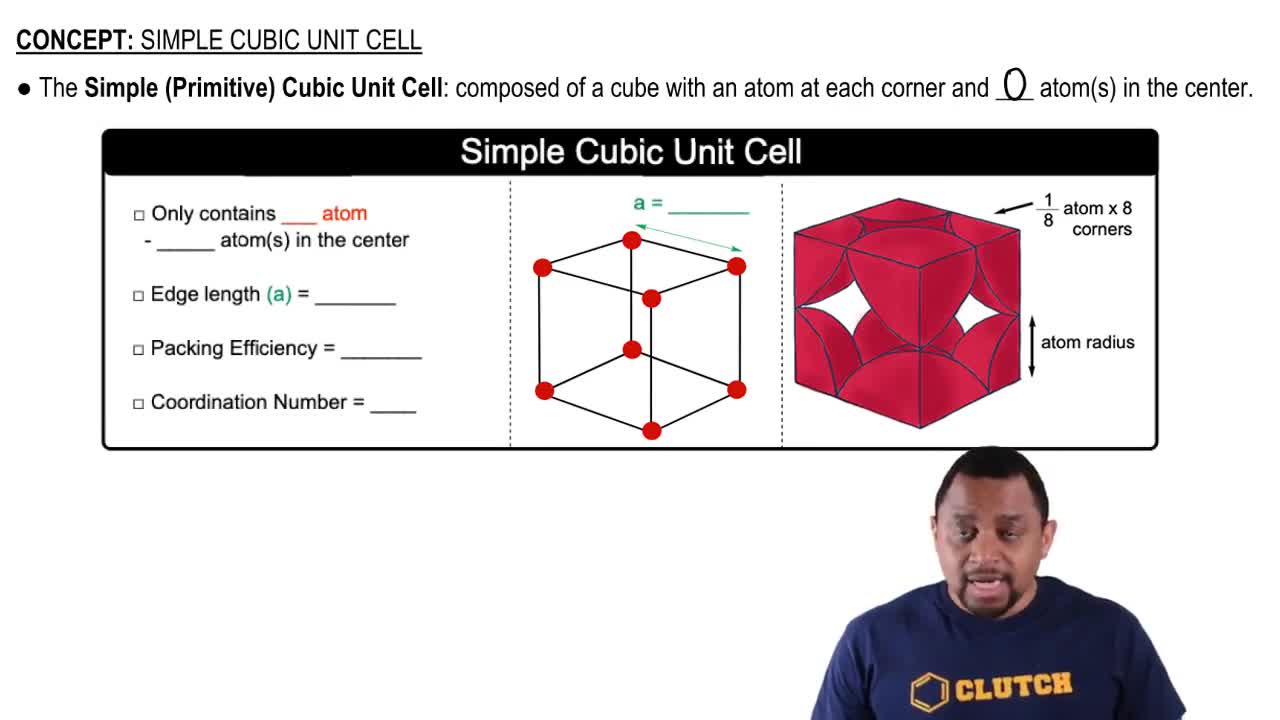
Simple Cubic Unit Cell
Body-Centered Cubic (BCC) Lattice
A body-centered cubic lattice is a type of crystal structure where atoms are located at each corner of a cube and one atom is positioned at the center of the cube. This arrangement results in a specific coordination number and packing efficiency, which influences the physical properties of the material. In a BCC structure, the minimum number of atoms per unit cell is essential for calculating density and other characteristics.
Recommended video:
Guided course

Body Centered Cubic Example
Atomic Contribution in Unit Cells
The atomic contribution in unit cells refers to how many atoms effectively belong to a single unit cell based on their positions. In a BCC lattice, each corner atom contributes 1/8 of an atom to the unit cell, and the center atom contributes a full atom. This concept is vital for determining the total number of atoms in the unit cell and solving related problems in crystallography.
Recommended video:
Guided course

Simple Cubic Unit Cell
Related Practice
Textbook Question
Textbook Question
Which of the three-dimensional primitive lattices has a unit cell where none of the internal angles is 90? (a) Orthorhombic, (b) hexagonal, (c) rhombohedral, (d) triclinic, (e) both rhombohedral and triclinic.
Textbook Question
What is the minimum number of atoms that could be contained in the unit cell of an element with a face-centered cubic lattice? (a) 1, (b) 2, (c) 3, (d) 4, (e) 5.
Textbook Question
The unit cell of nickel arsenide is shown here. (b) What is the empirical formula?
Textbook Question
The unit cell of a compound containing potassium, aluminum, and fluorine is shown here. (a) What type of lattice does this crystal possess (all three lattice vectors are mutually perpendicular)?
