Which of the following statements does not follow from the fact that the alkali metals have relatively weak metal–metal bonding? (a) The alkali metals are less dense than other metals. (b) The alkali metals are soft enough to be cut with a knife. (c) The alkali metals are more reactive than other metals. (d) The alkali metals have higher melting points than other metals. (e) The alkali metals have low ionization energies.

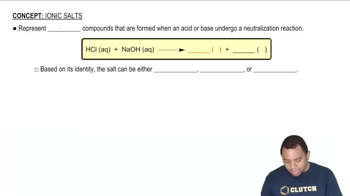
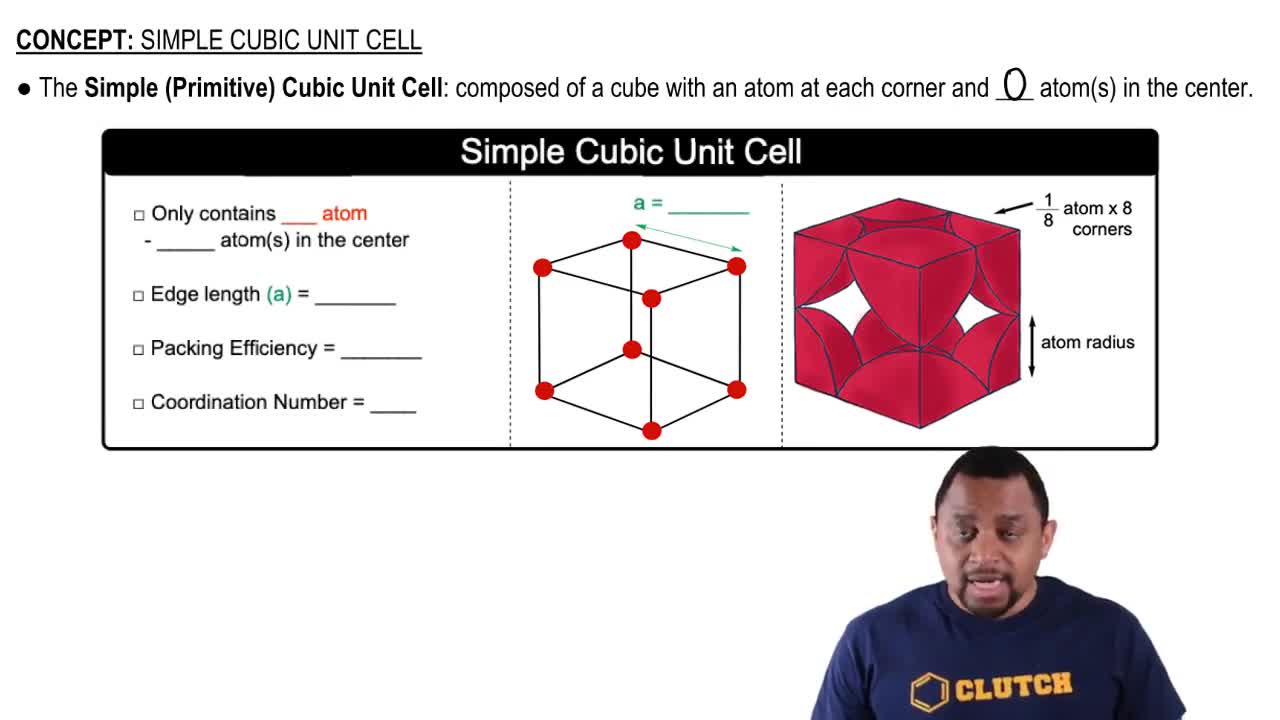
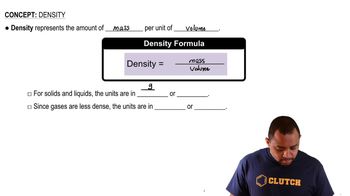
Alabandite is a mineral composed of manganese(II) sulfide (MnS). The mineral adopts the rock salt structure. The length of an edge of the MnS unit cell is 5.223 Å at 25 °C. Determine the density of MnS in g/cm³.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Rock Salt Structure
Unit Cell and Edge Length
Density Calculation
Tausonite, a mineral composed of Sr, O, and Ti, has the cubic unit cell shown in the drawing. (a) What is the empirical formula of this mineral?
The unit cell of a compound containing Co and O has a unit cell shown in the diagram. The Co atoms are on the corners, and the O atoms are completely within the unit cell. a. What is the empirical formula of this compound? b. What is the oxidation state of the metal?
A particular form of cinnabar (HgS) adopts the zinc blende structure. The length of the unit cell edge is 5.852 Å. (a) Calculate the density of HgS in this form. (c) Which of the two substances has the higher density? How do you account for the difference in densities?
A particular form of cinnabar (HgS) adopts the zinc blende structure. The length of the unit cell edge is 5.852 Å. (b) The mineral tiemannite (HgSe) also forms a solid phase with the zinc blende structure. The length of the unit cell edge in this mineral is 6.085 Å. What accounts for the larger unit cell length in tiemmanite?
