At 25°C gallium is a solid with a density of 5.91 g/cm3 and a melting point, 29.8°C, just slightly above room temperature. The density of liquid gallium just above the melting point is 6.1 g/cm3. Based on this information, what unusual feature would you expect to find in the phase diagram of gallium?
Ch.11 - Liquids and Intermolecular Forces

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 11, Problem 73
In all four liquid crystalline phases shown in Figure 11.32, the long axis of the molecule preferentially orders along one or more specific directions. In three of the four phases the molecules also lose some freedom of translational motion. In which of the four liquid crystalline phases do the molecules retain the freedom to move in all three directions that they possess in the liquid phase: nematic, smectic A, smectic C, or cholesteric?
 Verified step by step guidance
Verified step by step guidance1
Understand the characteristics of each liquid crystalline phase: Nematic, Smectic A, Smectic C, and Cholesteric.
Nematic phase: Molecules are oriented in the same direction but have no positional order, allowing them to move freely in all directions.
Smectic A and Smectic C phases: Molecules are organized into layers, restricting their movement to within the layers, thus losing some translational freedom.
Cholesteric phase: Similar to the nematic phase but with a helical twist, which can affect the freedom of movement.
Identify that the nematic phase is the one where molecules retain the freedom to move in all three directions, similar to the liquid phase.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Liquid Crystalline Phases
Liquid crystals are materials that exhibit properties between those of conventional liquids and solid crystals. They can flow like a liquid but have some degree of molecular order, which can be categorized into different phases, such as nematic, smectic, and cholesteric. Each phase has distinct characteristics regarding molecular orientation and movement.
Recommended video:
Guided course
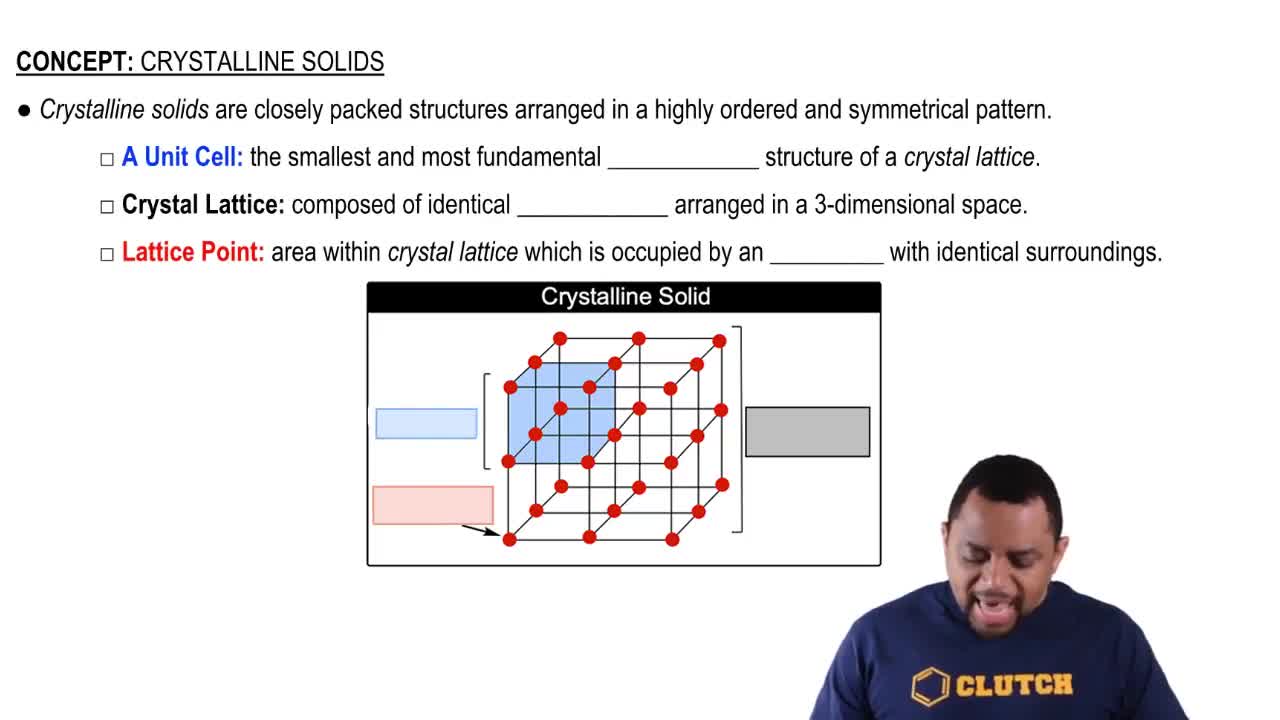
Crystalline Solids Structure
Nematic Phase
In the nematic phase, the long axes of the molecules are aligned in a preferred direction, but they retain the ability to move freely in the plane perpendicular to this direction. This means that while there is some order in molecular orientation, the molecules can still translate freely, similar to a liquid, allowing for fluidity and responsiveness to external forces.
Recommended video:
Guided course
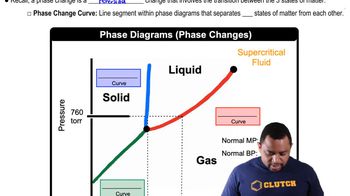
Phase Changes in Diagrams
Smectic Phase
The smectic phase is characterized by a higher degree of order compared to the nematic phase, where molecules are arranged in layers. In smectic A, the molecules are aligned within the layers but can slide past one another, while in smectic C, there is a tilt in the molecular arrangement. This layered structure restricts translational motion, unlike the nematic phase where such freedom is maintained.
Recommended video:
Guided course

Phase Changes in Diagrams
Related Practice
Textbook Question
Textbook Question
Indicate whether each statement is true or false: (c) Molecules that exhibit a liquid crystalline phase do so at well-defined temperatures and pressures.
Textbook Question
As the intermolecular attractive forces between molecules increase in magnitude, do you expect each of the following to increase or decrease in magnitude? (g) critical temperature.
Textbook Question
The table below lists the density of O2 at various temperatures and at 1 atm. The normal melting point of O2 is 54 K.
(b) Over what temperature range is O2 a liquid?
