Indicate whether each statement is true or false: (c) Molecules that exhibit a liquid crystalline phase do so at well-defined temperatures and pressures.

As the intermolecular attractive forces between molecules increase in magnitude, do you expect each of the following to increase or decrease in magnitude? (g) critical temperature.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
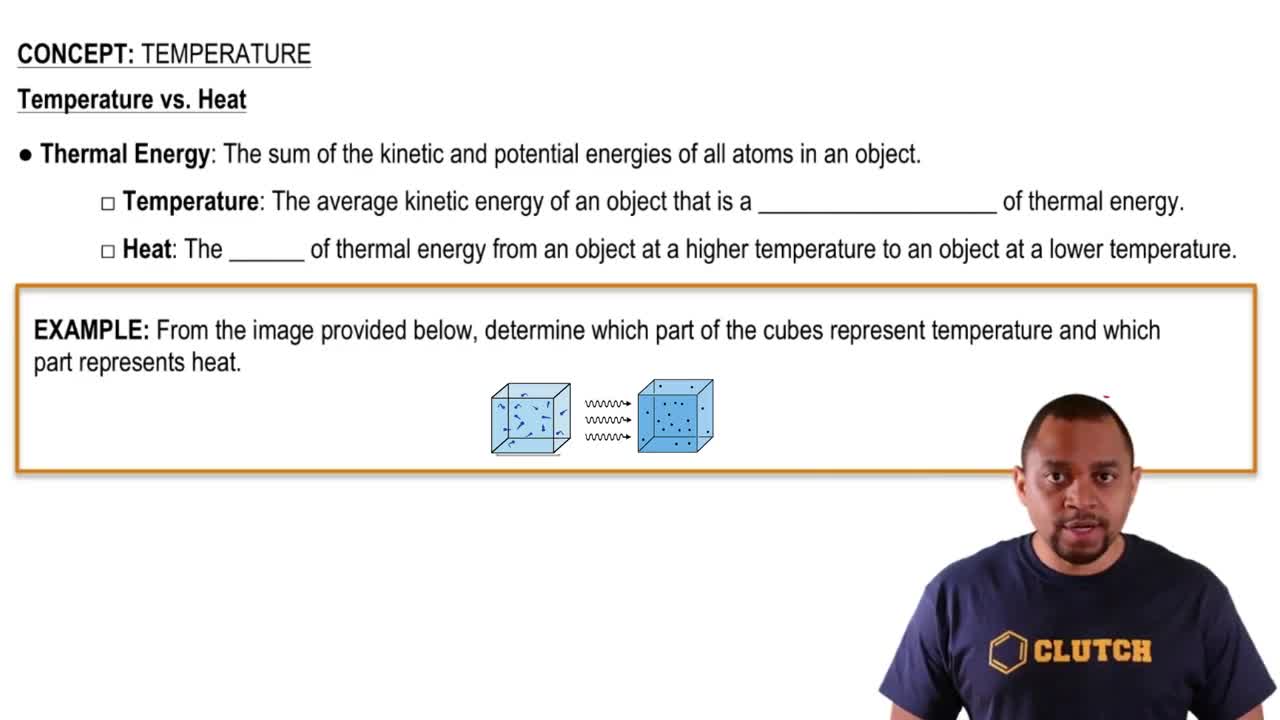
Key Concepts
Intermolecular Forces

Critical Temperature
Relationship Between Intermolecular Forces and Critical Temperature

In all four liquid crystalline phases shown in Figure 11.32, the long axis of the molecule preferentially orders along one or more specific directions. In three of the four phases the molecules also lose some freedom of translational motion. In which of the four liquid crystalline phases do the molecules retain the freedom to move in all three directions that they possess in the liquid phase: nematic, smectic A, smectic C, or cholesteric?
The table below lists the density of O2 at various temperatures and at 1 atm. The normal melting point of O2 is 54 K.
(b) Over what temperature range is O2 a liquid?
Suppose you have two colorless molecular liquids, one boiling at −84°C, the other at 34°C, and both at atmospheric pressure. Which of the following statements are true? d. The two liquids have identical vapor pressures at their normal boiling points.
Suppose you have two colorless molecular liquids, one boiling at - 84 °C, the other at 34 °C, and both at atmospheric 6 pressure. Which of the following statements is correct? For each statement that is not correct, modify the statement so that it is correct. (e) At - 84 °C both liquids have vapor pressures of 760 mm Hg.
