Ethyl chloride (C2H5Cl) boils at 12 °C. When liquid C2H5Cl under pressure is sprayed on a room-temperature (25 °C) surface in air, the surface is cooled considerably. (b) Assume that the heat lost by the surface is gained by ethyl chloride. What enthalpies must you consider if you were to calculate the final temperature of the surface?
Ch.11 - Liquids and Intermolecular Forces

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 11, Problem 47
Ethanol (C2H5OH) melts at -114 °C and boils at 78 °C. The enthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpy of vaporization is 38.56 kJ/mol. The specific heats of solid and liquid ethanol are 0.97 and 2.3 J/g-K, respectively. (a) How much heat is required to convert 42.0 g of ethanol at 35 °C to the vapor phase at 78 °C? (b) How much heat is required to convert the same amount of ethanol at -155 °C to the vapor phase at 78 °C?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Calculate the heat required to raise the temperature of ethanol from its initial temperature to its melting point. Use the formula q = m * c * ΔT, where m is the mass, c is the specific heat, and ΔT is the change in temperature.
Step 2: Calculate the heat required for the phase change from solid to liquid at the melting point using the enthalpy of fusion. Use the formula q = n * ΔH_fus, where n is the number of moles and ΔH_fus is the enthalpy of fusion.
Step 3: Calculate the heat required to raise the temperature of the liquid ethanol from the melting point to the boiling point. Again, use q = m * c * ΔT, but this time with the specific heat of liquid ethanol.
Step 4: Calculate the heat required for the phase change from liquid to vapor at the boiling point using the enthalpy of vaporization. Use the formula q = n * ΔH_vap, where ΔH_vap is the enthalpy of vaporization.
Step 5: Sum all the calculated heats from the previous steps to find the total heat required for the entire process.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Phase Changes and Enthalpy
Phase changes, such as melting and boiling, involve energy transfer in the form of enthalpy. The enthalpy of fusion is the heat required to convert a solid to a liquid at its melting point, while the enthalpy of vaporization is the heat needed to convert a liquid to a gas at its boiling point. Understanding these concepts is crucial for calculating the heat required during phase transitions.
Recommended video:
Guided course
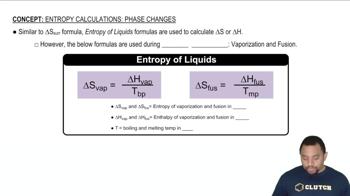
Entropy in Phase Changes
Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. Different phases of a substance have different specific heats, which affects the total heat calculation when changing temperature. For ethanol, the specific heats for solid and liquid phases are 0.97 J/g-K and 2.3 J/g-K, respectively, and are essential for determining heat changes during temperature adjustments.
Recommended video:
Guided course
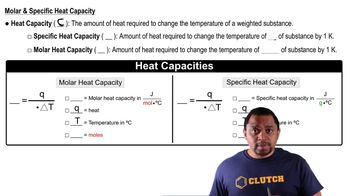
Heat Capacity
Heat Transfer Calculations
Heat transfer calculations involve using the formulas Q = mcΔT for temperature changes and Q = nΔH for phase changes, where Q is heat, m is mass, c is specific heat, n is the number of moles, and ΔH is the enthalpy change. These calculations are necessary to determine the total heat required for processes involving both temperature changes and phase transitions, as seen in the ethanol example.
Recommended video:
Guided course
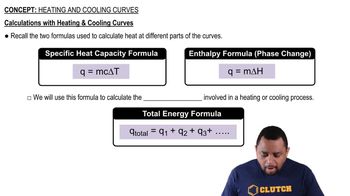
Calculations with Heating and Cooling Curves
Related Practice
Textbook Question
Textbook Question
For many years drinking water has been cooled in hot climates by evaporating it from the surfaces of canvas bags or porous clay pots. How many grams of water can be cooled from 35 to 20 °C by the evaporation of 60 g of water? (The heat of vaporization of water in this temperature range is 2.4 kJ/g. The specific heat of water is 4.18 J/g-K).
Textbook Question
The critical temperatures and pressures of a series of halogenated methanes are as follows:
(a) List the intermolecular forces that occur for each compound.
