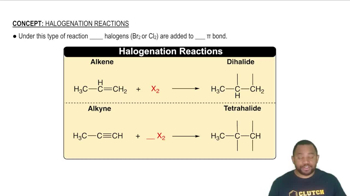
Textbook Question
The critical temperatures and pressures of a series of halogenated methanes are as follows: (c) Predict the critical temperature and pressure for CCl4 based on the trends in this table. Look up the experimentally determined critical temperatures and pressures for CCl4, using a source such as the CRC Handbook of Chemistry and Physics, and suggest a reason for any discrepancies.