(a) Which type of intermolecular attractive force operates between all molecules?

(b) Which of these kinds of interactions are broken when a liquid is converted to a gas?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
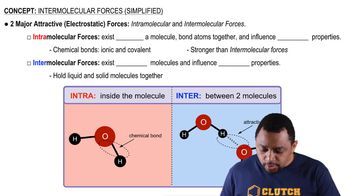
Key Concepts
Intermolecular Forces
Phase Change
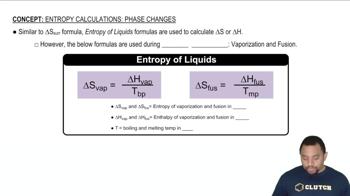
Vaporization
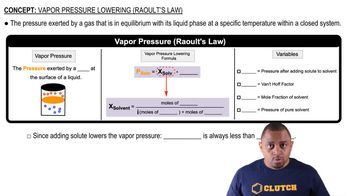
(b) Which type of intermolecular attractive force operates only between polar molecules?
(c) Which type of intermolecular attractive force operates only between the hydrogen atom of a polar bond and a nearby small electronegative atom?
Describe the intermolecular forces that must be overcome to convert these substances from a liquid to a gas: (a) SO2, (b) CH3COOH, (c) H2S.
Which type of intermolecular force accounts for each of these differences? (a) CH3OH boils at 65 °C; CH3SH boils at 6 °C. (d) Acetone boils at 56 °C, whereas 2-methylpropane boils at -12 °C.
Which type of intermolecular force accounts for each of these differences? (b) Xe is a liquid at atmospheric pressure and 120 K, whereas Ar is a gas under the same conditions. (c) Kr, atomic weight 84 amu, boils at 120.9 K, whereas Cl2, molecular weight about 71 amu, boils at 238 K.
