Ethylene glycol (HOCH2CH2OH), the major substance in antifreeze, has a normal boiling point of 198 °C. By comparison, ethyl alcohol (CH3CH2OH) boils at 78 °C at atmospheric pressure. Ethylene glycol dimethyl ether (CH3OCH2CH2OCH3) has a normal boiling point of 83 °C, and ethyl methyl ether (CH3CH2OCH3) has a nomral boiling point of 11 °C. (b) What are the major factors responsible for the difference in boiling points of the two ethers?

A number of salts containing the tetrahedral polyatomic anion, BF4-, are ionic liquids, whereas salts containing the somewhat larger tetrahedral ion SO42- do not form ionic liquids. Explain this observation.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ionic Liquids
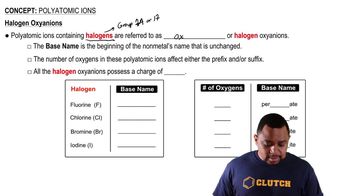
Polyatomic Anions
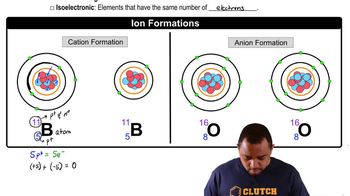
Ion Size and Packing
Based on the type or types of intermolecular forces, predict the substance in each pair that has the higher boiling point: (a) propane (C3H8) or n-butane (C4H10) (b) diethyl ether (CH3CH2OCH2CH3) or 1-butanol (CH3CH2CH2CH2OH) (c) sulfur dioxide (SO2) or sulfur trioxide (SO3) (d) phosgene (Cl2CO) or formaldehyde (H2CO)
The generic structural formula for a 1-alkyl-3-methylimid- azolium cation is where R is a -CH2(CH2)nCH3 alkyl group. The melting points of the salts that form between 1-alkyl-3-methylimidazolium cation and the PF6- anion are as follows: R = CH2CH3 (m.p. = 60 °C), R = CH2CH2CH3 (m.p. = 40 °C), r = CH2CH2CH2CH3 (m.p. = 10 °C), and R = CH2CH2CH2CH2CH2CH3 (m.p. = -61 °C). Why does the melting point decrease as the length of alkyl group increases?
(b) What is the relationship between viscosity and temperature?
(c) Why do substances with high surface tension also tend to have high viscosities?
