Arsenic(III) sulfide sublimes readily, even below its melting point of 320 °C. The molecules of the vapor phase are found to effuse through a tiny hole at 0.52 times the rate of effusion of Xe atoms under the same conditions of temperature and pressure. What is the molecular formula of arsenic(III) sulfide in the gas phase?

The planet Jupiter has a surface temperature of 140 K and a mass 318 times that of Earth. Mercury (the planet) has a surface temperature between 600 K and 700 K and a mass 0.05 times that of Earth. On which planet is the atmosphere more likely to obey the ideal-gas law? Explain.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
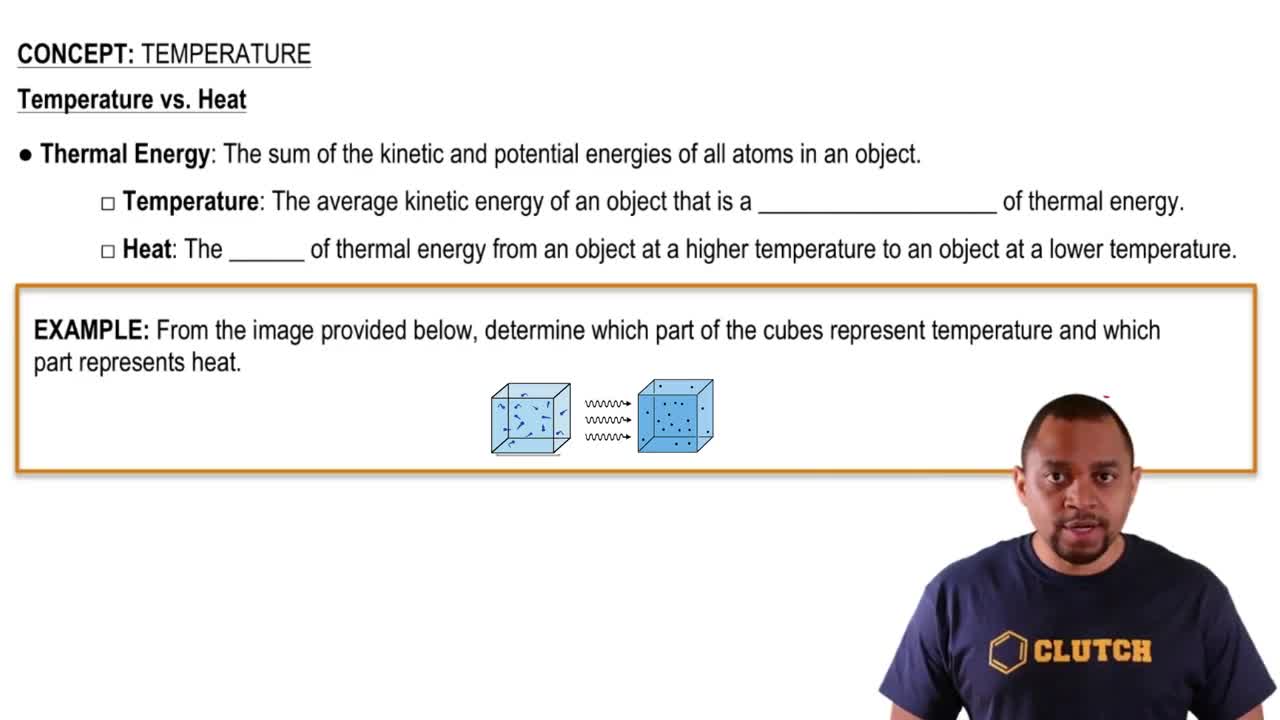
Key Concepts
Ideal Gas Law

Temperature and Gas Behavior
Atmospheric Composition and Pressure

A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 31 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas. (Remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 L; in other words, rate is the amount that diffuses over the time it takes to diffuse.)
(b) List two reasons why the gases deviate from ideal behavior.
Which statement concerning the van der Waals constants a and b is true? (a) The magnitude of a relates to molecular volume, whereas b relates to attractions between molecules. (b) The magnitude of a relates to attractions between molecules, whereas b relates to molecular volume. (c) The magnitudes of a and b depend on pressure. (d) The magnitudes of a and b depend on temperature.
Calculate the pressure that CCl4 will exert at 80 °C if 1.00 mol occupies 33.3 L, assuming that (a) CCl4 obeys the ideal-gas equation (b) CCl4 obeys the van der Waals equation. (Values for the van der Waals constants are given in Table 10.3.)
Table 10.3 shows that the van der Waals b parameter has units of L/mol. This means that we can calculate the sizes of atoms or molecules from the b parameter. Refer back to the discussion in Section 7.3. Is the van der Waals radius we calculate from the b parameter of Table 10.3 more closely associated with the bonding or nonbonding atomic radius discussed there? Explain.
