At constant pressure, the mean free path 1l2 of a gas molecule is directly proportional to temperature. At constant temperature, l is inversely proportional to pressure. If you compare two different gas molecules at the same temperature and pressure, l is inversely proportional to the square of the diameter of the gas molecules. Put these facts together to create a formula for the mean free path of a gas molecule with a proportionality constant (call it Rmfp, like the ideal-gas constant) and define units for Rmfp.

A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 31 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas. (Remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 L; in other words, rate is the amount that diffuses over the time it takes to diffuse.)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Graham's Law of Effusion
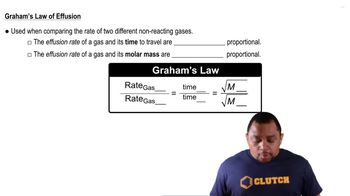
Molar Mass

Rate of Effusion
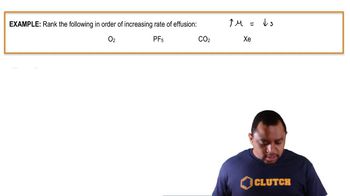
Hydrogen has two naturally occurring isotopes, 1H and 2H. Chlorine also has two naturally occurring isotopes, 35Cl and 37Cl. Thus, hydrogen chloride gas consists of four distinct types of molecules: 1H35Cl, 1H37Cl, 2H35Cl, and 2H37Cl. Place these four molecules in order of increasing rate of effusion.
Arsenic(III) sulfide sublimes readily, even below its melting point of 320 °C. The molecules of the vapor phase are found to effuse through a tiny hole at 0.52 times the rate of effusion of Xe atoms under the same conditions of temperature and pressure. What is the molecular formula of arsenic(III) sulfide in the gas phase?
(b) List two reasons why the gases deviate from ideal behavior.
The planet Jupiter has a surface temperature of 140 K and a mass 318 times that of Earth. Mercury (the planet) has a surface temperature between 600 K and 700 K and a mass 0.05 times that of Earth. On which planet is the atmosphere more likely to obey the ideal-gas law? Explain.
Which statement concerning the van der Waals constants a and b is true? (a) The magnitude of a relates to molecular volume, whereas b relates to attractions between molecules. (b) The magnitude of a relates to attractions between molecules, whereas b relates to molecular volume. (c) The magnitudes of a and b depend on pressure. (d) The magnitudes of a and b depend on temperature.
