You have a sample of gas at −33°C. You wish to increase the rms speed by a factor of 2. To what temperature should the gas be heated?

Consider the following gases, all at STP: Ne, SF6, N2, CH4. (f) Which one would effuse more rapidly than N2?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Graham's Law of Effusion
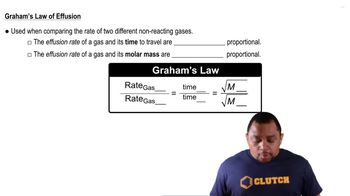
Molar Mass

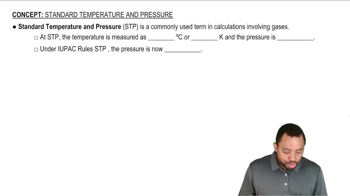
Standard Temperature and Pressure (STP)
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (a) Which gas is most likely to depart from the assumption of the kinetic-molecular theory that says there are no attractive or repulsive forces between molecules?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (d) Which one has the highest total molecular volume relative to the space occupied by the gas?
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (g) Which one would have the largest van der Waals b parameter?
Large amounts of nitrogen gas are used in the manufacture of ammonia, principally for use in fertilizers. Suppose 120.00 kg of N2(g) is stored in a 1100.0-L metal cylinder at 280 °C. (b) By using the data in Table 10.3, calculate the pressure of the gas according to the van der Waals equation.
