An ideal gas at a pressure of 1.50 atm is contained in a bulb of unknown volume. A stopcock is used to connect this bulb with a previously evacuated bulb that has a volume of 0.800 L as shown here. When the stopcock is opened, the gas expands into the empty bulb. If the temperature is held constant during this process and the final pressure is 695 torr, what is the volume of the bulb that was originally filled with gas?
Ch.10 - Gases

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 10, Problem 107d
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (d) Which one has the highest total molecular volume relative to the space occupied by the gas?
 Verified step by step guidance
Verified step by step guidance1
Understand that at STP (Standard Temperature and Pressure), all gases occupy the same volume per mole, which is 22.4 L.
Recognize that the total molecular volume of a gas is related to the size of its molecules and the number of molecules present.
Consider that the molecular volume of a gas is negligible compared to the volume it occupies at STP, but larger molecules will have a slightly higher molecular volume.
Compare the molecular sizes: Ne (neon) is a noble gas with small atomic size, SF_6 (sulfur hexafluoride) is a large molecule due to its six fluorine atoms, N_2 (nitrogen) is a diatomic molecule, and CH_4 (methane) is a small tetrahedral molecule.
Conclude that SF_6, being the largest molecule among the given gases, will have the highest total molecular volume relative to the space occupied by the gas.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law (PV=nRT) describes the relationship between pressure (P), volume (V), temperature (T), and the number of moles (n) of a gas. At standard temperature and pressure (STP), gases behave ideally, allowing for the comparison of their volumes. Understanding this law is crucial for analyzing how different gases occupy space under the same conditions.
Recommended video:
Guided course

Ideal Gas Law Formula
Molar Volume of Gases
At STP, one mole of an ideal gas occupies a volume of 22.4 liters. This concept allows for the comparison of the volumes of different gases based on their molar masses and molecular structures. Gases with larger molar masses or more complex structures may occupy more space, influencing their total molecular volume.
Recommended video:
Guided course
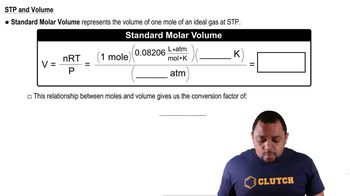
Standard Molar Volume
Density of Gases
The density of a gas is defined as its mass per unit volume. It is influenced by the molecular weight and the conditions of temperature and pressure. In the context of the question, understanding the densities of Ne, SF6, N2, and CH4 helps determine which gas has the highest total molecular volume relative to the space it occupies, as denser gases will have more mass in the same volume.
Recommended video:
Guided course

Density Concepts
Related Practice
Textbook Question
Textbook Question
You have a sample of gas at −33°C. You wish to increase the rms speed by a factor of 2. To what temperature should the gas be heated?
Textbook Question
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (a) Which gas is most likely to depart from the assumption of the kinetic-molecular theory that says there are no attractive or repulsive forces between molecules?
Textbook Question
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (f) Which one would effuse more rapidly than N2?
Textbook Question
Consider the following gases, all at STP: Ne, SF6, N2, CH4. (g) Which one would have the largest van der Waals b parameter?
