You have a sample of gas in a container with a movable piston, such as the one in the drawing. b. Redraw the container to show what it might look like if the external pressure on the piston is increased from 1.0 atm to 2.0 atm while the temperature is kept constant.

The apparatus shown here has two gas-filled containers and one empty container, all attached to a hollow horizontal tube closed at both ends.
a. How many blue gas molecules are in the left container?
b. How many red gas molecules are in the middle container?
c. When the valves are opened and the gases are allowed to mix at constant temperature, how many atoms of each type of gas end up in the originally empty container? Assume that the containers are of equal volume and ignore the volume of the connecting tube. [Section 10.4]
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
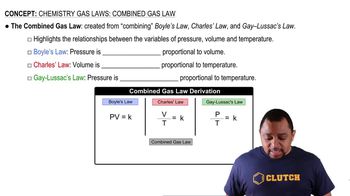
Gas Laws
Mole Concept

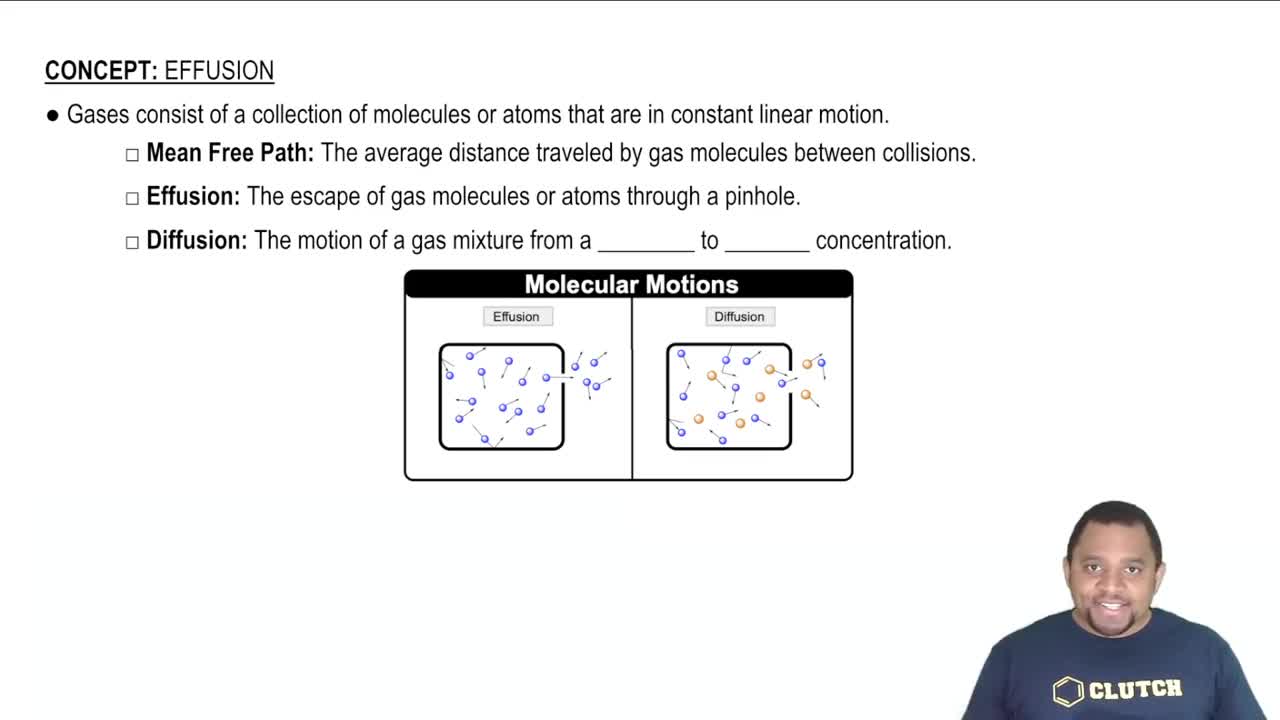
Diffusion of Gases
Consider the sample of gas depicted here. What would the drawing look like if the volume and temperature remained constant while you removed enough of the gas to decrease the pressure by a factor of 2? (a) It would contain the same number of molecules. (b) It would contain half as many molecules. (c) It would contain twice as many molecules. (d) There is insufficient data to say.
Imagine that the reaction 2 CO1g2 + O21g2¡2 CO21g2 occurs in a container that has a piston that moves to maintain a constant pressure when the reaction occurs at constant temperature. Which of the following statements describes how the volume of the container changes due to the reaction: (a) the volume increases by 50%, (b) the volume increases by 33%, (c) the volume remains constant, (d) the volume decreases by 33%, (e) the volume decreases by 50%.
Consider the following graph. (a) If curves A and B refer to two different gases, He and O2, at the same temperature, which curve corresponds to He?
Consider the following graph. (b) If A and B refer to the same gas at two different temperatures, which represents the higher temperature?
