Textbook Question
Two spheres of equal volume are placed on the scales as shown. a. Which one is more dense?

 Verified step by step guidance
Verified step by step guidance
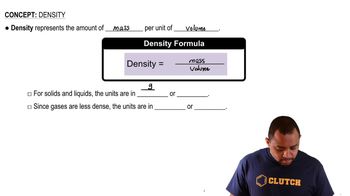
Two spheres of equal volume are placed on the scales as shown. a. Which one is more dense?
A 32.65-g sample of a solid is placed in a flask. Toluene, in which the solid is insoluble, is added to the flask so that the total volume of solid and liquid together is 50.00 mL. The solid and toluene together weigh 58.58 g. The density of toluene at the temperature of the experiment is 0.864 g/mL. What is the density of the solid?
Automobile batteries contain sulfuric acid, which is commonly referred to as “battery acid.” Calculate the number of grams of sulfuric acid in 1.00 gal of battery acid if the solution has a density of 1.28 g/mL and is 38.1% sulfuric acid by mass.