Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (a) Calculate the partial pressure of ozone at 441 ppb if the atmospheric pressure is 0.67 atm.

The average concentration of carbon monoxide in the air in an Ohio city in 2006 was 3.5 ppm. Calculate the number of CO molecules in 1.0 L of this air at a pressure of 759 torr and a temperature of 22 °C.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
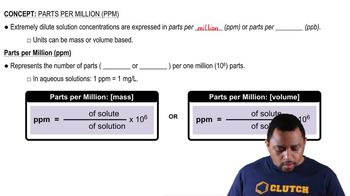
Parts Per Million (ppm)
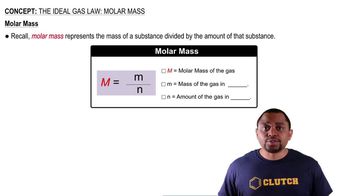
Ideal Gas Law

Molar Volume of a Gas
Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (b) How many ozone molecules are in 1.0 L of air in Mexico City? Assume T = 25 °C.
From the data in Table 18.1, calculate the partial pressures of carbon dioxide and argon when the total atmospheric pressure is 1.05 bar.
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (b) Which kind of electromagnetic radiation—ultraviolet, visible, or infrared—does the wavelength you calculated in part (a) correspond to?
