An important reaction in the formation of photochemical smog is the photodissociation of NO : NO2 + hv → NO(g) + O(g) The maximum wavelength of light that can cause this reaction is 420 nm. (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 420-nm light?

Phosphorus is present in seawater to the extent of 0.07 ppm by mass. Assuming that the phosphorus is present as dihydrogenphosphate, H2PO4-, calculate the correspond-ing molar concentration of H2PO4- in seawater.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
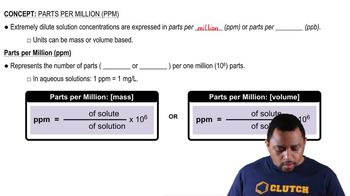
Key Concepts
Parts Per Million (ppm)
Molar Concentration

Molar Mass

What is the molarity of Na+ in a solution of NaCl whose salinity is 5.6 if the solution has a density of 1.03 g>mL?
The enthalpy of evaporation of water is 40.67 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (a) Assuming that evaporation of water is due only to energy input from the Sun, calculate how many grams of water could be evaporated from a 1.00 square meter patch of ocean over a 12-h day
The enthalpy of evaporation of water is 40.67 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (b) The specific heat capacity of liquid water is 4.184 J/g°C. If the initial surface temperature of a 1.00 square meter patch of ocean is 26 °C, what is its final temperature after being in sunlight for 12 h, assuming no phase changes and assuming that sunlight penetrates uniformly to depth of 10.0 cm?
The enthalpy of fusion of water is 6.01 kJ/mol. Sunlight striking Earth's surface supplies 168 W per square meter (1 W = 1 watt = 1 J/s). (b) The specific heat capacity of ice is 2.032 J/g°C. If the initial temperature of a 1.00 square emter patch of ice is -5.0°C, what is its final temperature after being in sunlight for 12 h, assuming no phase changes and assuming that sunlight penetration uniformly to a depth of 1.00 cm?
