Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reaction is 335 nm. (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 335-nm light?

An important reaction in the formation of photochemical smog is the photodissociation of NO : NO2 + hv → NO(g) + O(g) The maximum wavelength of light that can cause this reaction is 420 nm. (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 420-nm light?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
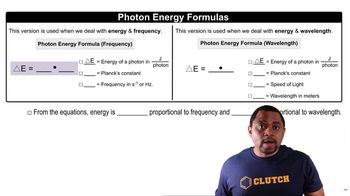
Key Concepts
Photodissociation
Photon Energy and Wavelength
Bond Strength

Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reac- tion is 335 nm. (d) Write out the formaldehyde photodis- sociation reaction, showing Lewis-dot structures.
An important reaction in the formation of photochemical smog is the photodissociation of NO : NO2 + hv → NO(g) + O(g) The maximum wavelength of light that can cause this reac- tion is 420 nm. (a) In what part of the electromagnetic spec- trum is light with this wavelength found?
What is the molarity of Na+ in a solution of NaCl whose salinity is 5.6 if the solution has a density of 1.03 g>mL?
Phosphorus is present in seawater to the extent of 0.07 ppm by mass. Assuming that the phosphorus is present as dihydrogenphosphate, H2PO4-, calculate the correspond-ing molar concentration of H2PO4- in seawater.
