Textbook Question
Show how Equations 18.7 and 18.9 can be added to give Equation 18.10.

 Verified step by step guidance
Verified step by step guidance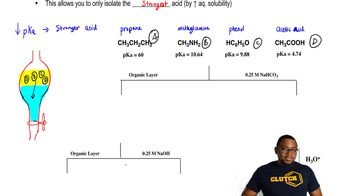


Show how Equations 18.7 and 18.9 can be added to give Equation 18.10.
(a) What is the difference between a CFC and an HFC?
Natural gas consists primarily of methane, CH4(g). (a) Write balanced chemical equation for the complete combustion of methane to produce CO2(g) as the only carbon-containing product.