Write balanced chemical equations for each of the following reactions: (a) The nitric oxide molecule undergoes photodissociation in the upper atmosphere. (b) The nitric oxide molecule undergoes photoionization in the upper atmosphere. (c) Nitric oxide undergoes oxidation by ozone in the stratosphere.
Ch.18 - Chemistry of the Environment

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 18, Problem 75b
(b) Will Mg(OH)2 precipitate when 4.0 g of Na2CO3 is added to 1.00 L of a solution containing 125 ppm of Mg2+?
 Verified step by step guidance
Verified step by step guidance1
Convert the concentration of Mg^{2+} from ppm to molarity. Since 125 ppm is equivalent to 125 mg/L, convert this to moles by dividing by the molar mass of Mg (24.31 g/mol).
Calculate the moles of Na2CO3 added by converting 4.0 g of Na2CO3 to moles using its molar mass (105.99 g/mol).
Determine the concentration of CO3^{2-} ions in the solution after adding Na2CO3. Since the volume of the solution is 1.00 L, the concentration is equal to the moles of Na2CO3.
Use the solubility product constant (K_{sp}) for Mg(OH)2 to determine the ion product (Q) of Mg^{2+} and OH^{-} in the solution. The K_{sp} expression for Mg(OH)2 is K_{sp} = [Mg^{2+}][OH^{-}]^2.
Compare the ion product (Q) to the K_{sp} value for Mg(OH)2. If Q > K_{sp}, Mg(OH)2 will precipitate; if Q < K_{sp}, it will not.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
14mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility Product Constant (Ksp)
The solubility product constant (Ksp) is a numerical value that represents the equilibrium between a solid and its ions in a saturated solution. For Mg(OH)2, Ksp indicates the maximum concentration of Mg2+ and OH- ions that can exist in solution before precipitation occurs. Understanding Ksp is essential to determine if the concentration of ions exceeds this value, leading to precipitation.
Recommended video:
Guided course

Solubility Product Constant
Precipitation Reactions
Precipitation reactions occur when two soluble salts react to form an insoluble compound, or precipitate. In this case, adding Na2CO3 introduces carbonate ions, which can react with Mg2+ ions to form Mg(OH)2. Recognizing the conditions under which precipitation occurs is crucial for predicting whether a solid will form in a solution.
Recommended video:
Guided course
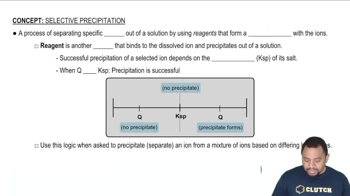
Selective Precipitation
Concentration Calculations
Concentration calculations involve determining the molarity of ions in a solution, which is essential for assessing whether a precipitate will form. In this scenario, converting the mass of Na2CO3 to moles and then calculating the resulting concentration of Mg2+ ions allows for comparison with Ksp. Accurate concentration calculations are vital for predicting the outcome of the reaction.
Recommended video:
Guided course
Calculate Concentration of the Basic Form
Related Practice
Textbook Question
1
views
Textbook Question
Write balanced chemical equations for each of the following reactions: (d) Nitrogen dioxide dissolves in water to form nitric acid and nitric oxide.
Textbook Question
(a) The EPA threshold for acceptable levels of lead ions in water is 615 ppb. What is the molarity of an aqueous solution with a concentration of 15 ppb?
Textbook Question
(b) Concentrations of lead in the bloodstream are often quoted in units of μg/dL. Averaged over the entire country, the mean concentration of lead in the blood was measured to be 1.6 μg/dL in 2008. Express this concentration in ppb.
