The Ogallala aquifer described in the Closer Look box in Section 18.3, provides 82% of the drinking water for the people who live in the region, although more than 75% of the water that is pumped from it is for irrigation. Irrigation withdrawals are approximately 18 billion gallons per day. (a) Assuming that 2% of the rainfall that falls on an area of 600,000 km2 recharges the aquifer, what average annual rainfall would be required to replace the water removed for irrigation?

Assume that a portable reverse-osmosis apparatus operates on seawater, whose concentrations of constituent ions are listed in Table 18.5, and that the desalinated water output has an effective molarity of about 0.02 M. What minimum pressure must be applied by hand pumping at 297 K to cause reverse osmosis to occur? (Hint: Refer to Section 13.5.)
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Reverse Osmosis

Osmotic Pressure
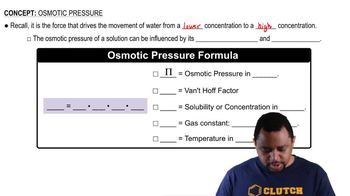
Ideal Gas Constant (R)

The Ogallala aquifer described in the Closer Look box in Section 18.3, provides 82% of the drinking water for the people who live in the region, although more than 75% of the water that is pumped from it is for irrigation. Irrigation withdrawals are approximately 18 billion gallons per day. (b) What process or processes accounts for the presence of arsenic in well water?
The organic anion
is found in most detergents. Assume that the anion under-goes aerobic decomposition in the following manner: C18H29SO3- + 51 O2 → 36 CO2(aq) + 28 H2O (l) + 2 H+(aq) + 2 SO42-(aq) What is the total mass of O2 required to biodegrade 10.0 g of this substance?
Magnesium ions are removed in water treatment by the addition of slaked lime, Ca(OH)2. Write a balanced chemical equation to describe what occurs in this process
