In the lime soda process once used in large scale munici-pal water softening, calcium hydroxide prepared from lime and sodium carbonate are added to precipitate Ca2+ as CaCO3(s) and Mg2+ as Mg(OH)2(s): Ca2+(aq) + CO32-(aq) → CaCO3(s) Mg2+(aq) + 2 OH-(aq) → MgOH2(aq) How many moles of Ca(OH)2 and Na2CO3 should be added to soften (remove the Ca2+ and Mg2+) 1200 L of water in which [Ca2+] = 5.0x10-4 M and [Mg2+] = 7.0x10-4 M?
Ch.18 - Chemistry of the Environment

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 18, Problem 51
Magnesium ions are removed in water treatment by the addition of slaked lime, Ca(OH)2. Write a balanced chemical equation to describe what occurs in this process
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants involved in the reaction: magnesium ions (Mg^{2+}) and slaked lime (Ca(OH)_2).
Determine the products formed when magnesium ions react with slaked lime. Magnesium ions will react with hydroxide ions (OH^-) to form magnesium hydroxide (Mg(OH)_2), which is insoluble in water and precipitates out.
Write the unbalanced chemical equation for the reaction: Mg^{2+} + Ca(OH)_2 \rightarrow Mg(OH)_2 + Ca^{2+}.
Balance the chemical equation by ensuring the number of atoms of each element is the same on both sides of the equation. In this case, the equation is already balanced as written.
Verify that the charges are balanced on both sides of the equation. The total charge on the left side is +2 (from Mg^{2+}), and the total charge on the right side is also +2 (from Ca^{2+}).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Reactions
A chemical reaction involves the transformation of reactants into products through the breaking and forming of chemical bonds. In the context of water treatment, understanding how magnesium ions interact with slaked lime is essential to write a balanced equation that accurately represents the process.
Recommended video:
Guided course

Chemical Properties
Balancing Chemical Equations
Balancing a chemical equation ensures that the number of atoms for each element is the same on both sides of the equation, adhering to the law of conservation of mass. This process involves adjusting coefficients to reflect the correct stoichiometry of the reactants and products involved in the reaction.
Recommended video:
Guided course

Balancing Chemical Equations
Slaked Lime in Water Treatment
Slaked lime, or calcium hydroxide (Ca(OH)2), is commonly used in water treatment to precipitate hardness-causing ions, such as magnesium (Mg²⁺). When added to water, it reacts with magnesium ions to form insoluble magnesium hydroxide, which can then be removed from the water, thus softening it.
Recommended video:
Guided course
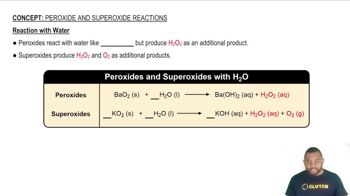
Reaction with Water
Related Practice
Textbook Question
Textbook Question
The organic anion
is found in most detergents. Assume that the anion under-goes aerobic decomposition in the following manner: C18H29SO3- + 51 O2 → 36 CO2(aq) + 28 H2O (l) + 2 H+(aq) + 2 SO42-(aq) What is the total mass of O2 required to biodegrade 10.0 g of this substance?
Textbook Question
(a) What are trihalomethanes (THMs)? (b) Draw the Lewis structures of two example THMs.
