(b) Will Mg(OH)2 precipitate when 4.0 g of Na2CO3 is added to 1.00 L of a solution containing 125 ppm of Mg2+?

As of the writing of this text, EPA standards limit atmospheric ozone levels in urban environments to 84 ppb. How many moles of ozone would there be in the air above Los Angeles County (area about 4000 square miles; consider a height of 100 m above the ground) if ozone was at this concentration?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
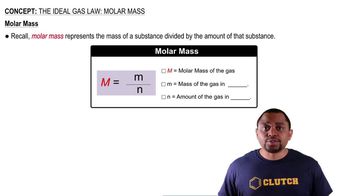
Molar Volume of a Gas
Concentration Units
Volume Calculation
(a) The EPA threshold for acceptable levels of lead ions in water is 615 ppb. What is the molarity of an aqueous solution with a concentration of 15 ppb?
(b) Concentrations of lead in the bloodstream are often quoted in units of μg/dL. Averaged over the entire country, the mean concentration of lead in the blood was measured to be 1.6 μg/dL in 2008. Express this concentration in ppb.
The estimated average concentration of NO2 in air in the United States in 2006 was 0.016 ppm. (a) Calculate the partial pressure of the NO2 in a sample of this air when the atmospheric pressure is 755 torr (99.1 kPa).
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 tons of coal, a national record at that time. (a) Assuming that the coal was 83% carbon and 2.5% sulfur and that combustion was complete, calculate the number of tons of carbon dioxide and sulfur dioxide produced by the plant during the year.
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 tons of coal, a national record at that time. (b) If 55% of the SO2 could be removed by reaction with powdered CaO to form CaSO3, how many tons of CaSO3 would be produced?
