(a) Write a chemical equation that describes the attack of acid rain on limestone, CaCO3.

Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photochemical smog: CH2O + hn → CHO + H. The maximum wavelength of light that can cause this reaction is 335 nm. (c) Compare your answer from part (b) to the appropriate value from Table 8.3. What do you conclude about the C−H bond energy in formaldehyde?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Photodissociation
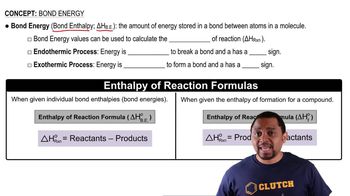
Bond Energy
Photochemical Smog
(b) If a limestone sculpture were treated to form a surface layer of calcium sulfate, would this help to slow down the effects of acid rain? Explain.
Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reaction is 335 nm. (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 335-nm light?
Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reac- tion is 335 nm. (d) Write out the formaldehyde photodis- sociation reaction, showing Lewis-dot structures.
An important reaction in the formation of photochemical smog is the photodissociation of NO : NO2 + hv → NO(g) + O(g) The maximum wavelength of light that can cause this reac- tion is 420 nm. (a) In what part of the electromagnetic spec- trum is light with this wavelength found?
