Textbook Question
(b) Based on average bond enthalpies, would you expect a photon capable of dissociating a C-Cl bond to have sufficient energy to dissociate a C-Br bond?

 Verified step by step guidance
Verified step by step guidance
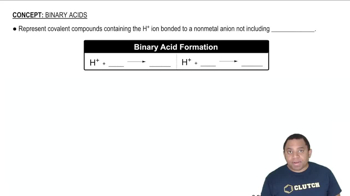
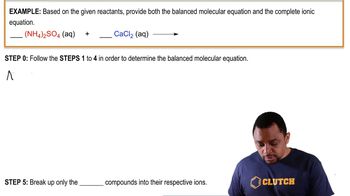

(b) Based on average bond enthalpies, would you expect a photon capable of dissociating a C-Cl bond to have sufficient energy to dissociate a C-Br bond?
(a) Write a chemical equation that describes the attack of acid rain on limestone, CaCO3.
Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reaction is 335 nm. (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 335-nm light?