Textbook Question
Consider the SCl2 molecule.(d) What valence orbitals, if any, remain unhybridized on the S atom in SCl2?

 Verified step by step guidance
Verified step by step guidance
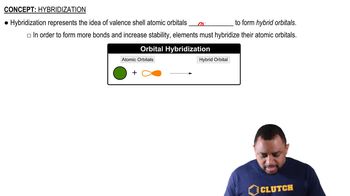
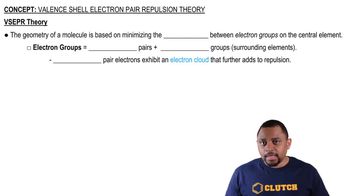
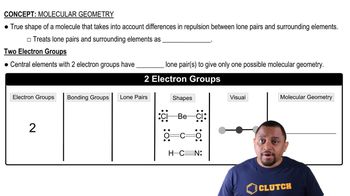
Consider the SCl2 molecule.(d) What valence orbitals, if any, remain unhybridized on the S atom in SCl2?
Indicate the hybridization of the central atom in (a) H2S (b) SeF6
Indicate the hybridization of the central atom in (c) P1OH23 (d) AlI3.
What is the hybridization of the central atom in (c) O3?
What is the hybridization of the central atom in (d) NO2?
Shown here are three pairs of hybrid orbitals, with each set at a characteristic angle. For each pair, determine the type of hybridization, if any, that could lead to hybrid orbitals at the specified angle. (a)
(b)
(c)