Sulfur tetrafluoride 1SF42 reacts slowly with O2 to form sulfur tetrafluoride monoxide 1OSF42 according to the following unbalanced reaction: SF41g2 + O21g2¡OSF41g2 The O atom and the four F atoms in OSF4 are bonded to a central S atom. (a) Balance the equation.
Ch.9 - Molecular Geometry and Bonding Theories

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 9, Problem 114e
Sulfur tetrafluoride 1SF42 reacts slowly with O2 to form sulfur
tetrafluoride monoxide 1OSF42 according to the following
unbalanced reaction:
SF41g2 + O21g2¡OSF41g2
The O atom and the four F atoms in OSF4 are bonded to a
central S atom.
(e) For each of the molecules you drew in part (d), state how many
fluorines are equatorial and how many are axial.
 Verified step by step guidance
Verified step by step guidance1
Identify the molecular geometry of SF_4. SF_4 has a seesaw shape due to the presence of one lone pair on the sulfur atom.
In a seesaw geometry, there are two types of positions for the fluorine atoms: equatorial and axial.
Determine the positions of the fluorine atoms in SF_4. In a seesaw shape, there are typically two equatorial positions and two axial positions.
Now, consider the molecular geometry of OSF_4. The presence of an additional oxygen atom bonded to sulfur may alter the geometry slightly, but the basic seesaw shape remains.
For OSF_4, identify the positions of the fluorine atoms. Typically, the equatorial positions are occupied first, so expect two equatorial and two axial fluorines.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influences the shape of the molecule. For sulfur tetrafluoride (SF4), the geometry is based on the VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts that the molecule adopts a seesaw shape due to the presence of one lone pair.
Recommended video:
Guided course

Molecular Geometry with Two Electron Groups
Equatorial and Axial Positions
In molecules with a trigonal bipyramidal geometry, such as SF4, the positions of atoms can be classified as equatorial or axial. Equatorial positions are located in the plane of the molecule and are 120 degrees apart, while axial positions are aligned above and below the equatorial plane at 90 degrees to the equatorial atoms. Understanding these positions is crucial for predicting molecular behavior and reactivity.
Recommended video:
Guided course
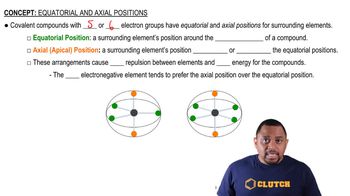
Equatorial vs. Axial Positions
Bonding and Hybridization
Bonding in molecules involves the overlap of atomic orbitals to form covalent bonds. In SF4, sulfur undergoes sp3d hybridization, which allows it to form four equivalent bonds with fluorine atoms and accommodate one lone pair. This hybridization is essential for understanding the molecule's geometry and the distribution of electron density around the central sulfur atom.
Recommended video:
Guided course

Hybridization
Related Practice
Textbook Question
Textbook Question
Sulfur tetrafluoride (SF4) reacts slowly with O2 to form sulfur tetrafluoride monoxide (OSF4) according to the following unbalanced reaction: SF4(g) + O2(g) → OSF4(g) The O atom and the four F atoms in OSF4 are bonded to a central S atom. (b) Write a Lewis structure of OSF4 in which the formal charges of all atoms are zero.
Textbook Question
Sulfur tetrafluoride (SF4) reacts slowly with O2 to form sulfur tetrafluoride monoxide (OSF4) according to the following unbalanced reaction: SF4(g) + O2(g) → OSF4(g) The O atom and the four F atoms in OSF4 are bonded to a central S atom. (c) Use average bond enthalpies (Table 8.3) to estimate the enthalpy of the reaction. Is it endothermic or exothermic?
Textbook Question
The phosphorus trihalides 1PX32 show the following variation in the bond angle X¬P¬X: PF3, 96.3°; PCl3, 100.3°; PBr3, 101.0°; PI3, 102.0°. The trend is generally attributed to the change in the electronegativity of the halogen. (b) What is the general trend in the X¬P¬X angle as the halide electronegativity increases?
