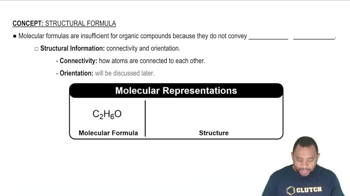
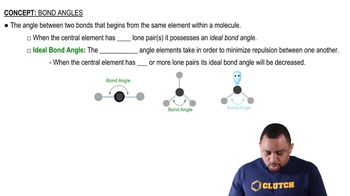
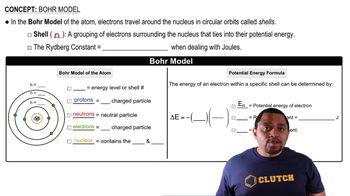
Textbook Question
The organic molecules shown here are derivatives of benzenein which six-membered rings are 'fused' at the edgesof the hexagons.(e) Benzene, naphthalene, and anthraceneare colorless, but tetracene is orange. What does this implyabout the relative HOMO–LUMO energy gaps in these molecules?See the 'Chemistry Put to Work' box on orbitalsand energy.