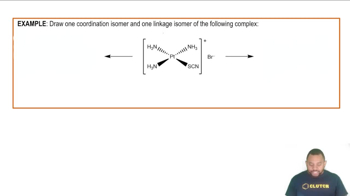
Textbook Question
Many compounds of the transition-metal elements containdirect bonds between metal atoms. We will assumethat the z-axis is defined as the metal–metal bond axis.(d) Sketch the energyleveldiagram for the Sc2 molecule, assuming that only the3d orbital from part (a) is important.
1
views