Textbook Question
(b) How would you expect the H¬X¬H bond angle to vary in the series H2O, H2S, H2Se? Explain. (Hint: The size of an electron pair domain depends in part on the electronegativity of the central atom.)

 Verified step by step guidance
Verified step by step guidance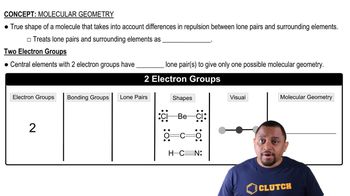
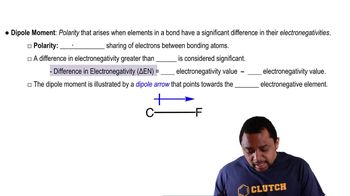
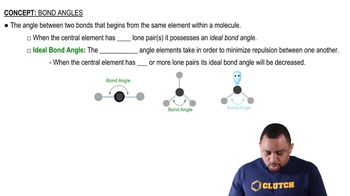
(b) How would you expect the H¬X¬H bond angle to vary in the series H2O, H2S, H2Se? Explain. (Hint: The size of an electron pair domain depends in part on the electronegativity of the central atom.)
(a) Does CS2 have a dipole moment? If so, in which direction does the net dipole point? (b) Does SO2 have a dipole moment? If so, in which direction does the net dipole point?
(b) It turns out that ozone, O3, has a small dipole moment. How is this possible, given that all the atoms are the same?