One scale for electronegativity is based on the concept that the electronegativity of any atom is proportional to the ionization energy of the atom minus its electron affinity: electronegativity = k1I - EA2, where k is a proportionality constant. (b) Why are both ionization energy and electron affinity relevant to the notion of electronegativity?

The compound chloral hydrate, known in detective stories as knockout drops, is composed of 14.52% C, 1.83% H, 64.30% Cl, and 13.35% O by mass, and has a molar mass of 165.4 g/mol. (c) Draw the Lewis structure of the molecule, assuming that the Cl atoms bond to a single C atom and that there are a C–C bond and two C–O bonds in the compound.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Molecular Composition and Percent Composition
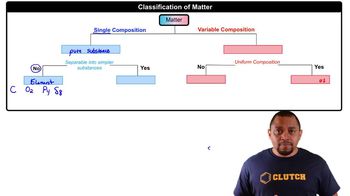
Molar Mass and Molecular Formula

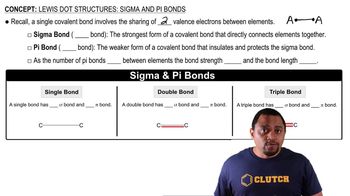
Lewis Structures and Bonding
One scale for electronegativity is based on the concept that the electronegativity of any atom is proportional to the ionization energy of the atom minus its electron affinity: electronegativity = k1I - EA2, where k is a proportionality constant. (c) By using data in Chapter 7, determine the value of k that would lead to an electronegativity of 4.0 for F under this definition.
One scale for electronegativity is based on the concept that the electronegativity of any atom is proportional to the ionization energy of the atom minus its electron affinity: electronegativity = k1I - EA2, where k is a proportionality constant. (d) Use your result from part (c) to determine the electronegativities of Cl and O using this scale. Use your result to determine the electronegativity of Cl using this scale.
Acetylene (C2H2) and nitrogen (N2) both contain a triple bond, but they differ greatly in their chemical properties. (b) By referring to Appendix C, look up the enthalpies of formation of acetylene and nitrogen. Which compound is more stable?
Acetylene (C2H2) and nitrogen (N2) both contain a triple bond, but they differ greatly in their chemical properties. (c) Write balanced chemical equations for the complete oxidation of N2 to form N2O5(g) and of acetylene to form CO2(g) and H2O(g). Write a balanced chemical equation for the complete oxidation of acetylene to form CO2(g) and H2O(g).
