A portion of a two-dimensional 'slab' of NaCl(s) is shown here (see Figure 8.2) in which the ions are numbered. (d) Consider ion 5. How many repulsive interactions are shown for it?

Fill in the blank with the appropriate numbers for both electrons and bonds (considering that single bonds are counted as one, double bonds as two, and triple bonds as three). (a) Fluorine has _ valence electrons and makes _ bond(s) in compounds. (b) Oxygen has _ valence electrons and makes _ bond(s) in compounds. (c) Nitrogen has _ valence electrons and makes _ bond(s) in compounds. (d) Carbon has _ valence electrons and makes _ bond(s) in compounds.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
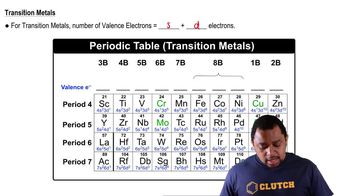
Valence Electrons
Types of Chemical Bonds
Octet Rule
The orbital diagram that follows shows the valence electrons for a 2+ ion of an element. (a) What is the element?
In the Lewis structure shown here, A, D, E, Q, X, and Z represent elements in the first two rows of the periodic table. Identify all six elements so that the formal charges of all atoms are zero.
The partial Lewis structure that follows is for a hydrocarbon molecule. In the full Lewis structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in the molecule. The carbon—carbon bonds are labeled 1, 2, and 3. (a) How many hydrogen atoms are in the molecule?
The partial Lewis structure that follows is for a hydrocarbon molecule. In the full Lewis structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in the molecule. The carbon—carbon bonds are labeled 1, 2, and 3. (c) Which carbon—carbon bond is the strongest one?
Consider the Lewis structure for the polyatomic oxyanion shown here, where X is an element from the third period (Na - Ar). By changing the overall charge, n, from 1- to 2- to 3- we get three different polyatomic ions. For each of these ions (b) determine the formal charge of the central atom, X;
